|
Heteroazeotrope
A heteroazeotrope is an azeotrope where the vapour phase coexists with two liquid phases. Sketch of a T-x/y equilibrium curve of a typical heteroazeotropic mixture Examples of heteroazeotropes *Benzene - Water NBP 69.2 °C *Dichloromethane - Water NBP 38.5 °C * n-Butanol - Water NBP 93.5 °C * Toluene - Water NBP 82 °C Continuous heteroazeotropic distillation Heterogeneous distillation means that during the distillation the liquid phase of the mixture is immiscible. In this case on the plates can be two liquid phases and the top vapour condensate splits in two liquid phases, which can be separated in a decanter. The simplest case of continuous heteroazeotropic distillation is the separation of a binary heterogeneous azeotropic mixture. In this case the system contains two columns and a decanter. The fresh feed (A-B) is added into the first column. (The feed may also be added into the decanter directly or into the second column depending on the composition of the mixture). Fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
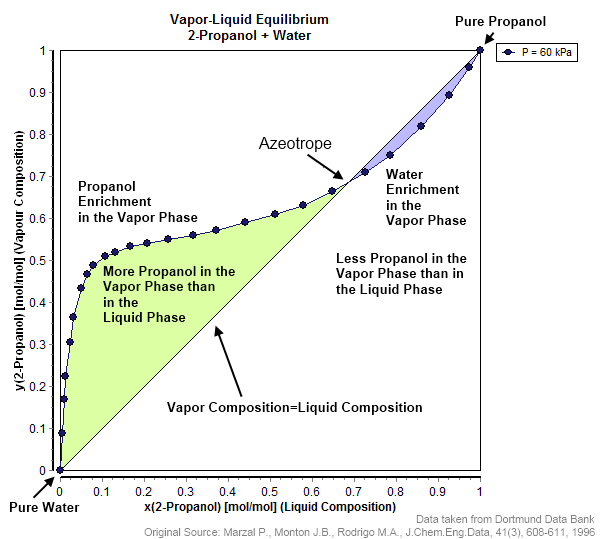
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Knowing an azeotrope's behavior is important for distillation. Each azeotrope has a characteristic boiling point. The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point of any of its constituents (a negative azeotrope). For both positive and negative azeotropes, it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. For technical applications, the pressure-temperature-composition behavior of a mixture is the most important, but other important ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Batch Distillation
Batch distillation refers to the use of distillation in batches, meaning that a mixture is distilled to separate it into its component fractions before the distillation still is again charged with more mixture and the process is repeated. This is in contrast with continuous distillation where the feedstock is added and the distillate drawn off without interruption. Batch distillation has always been an important part of the production of seasonal, or low capacity and high-purity chemicals. It is a very frequent separation process in the pharmaceutical industry. Batch rectifier The simplest and most frequently used batch distillation configuration is the batch rectifier, including the alembic and pot still. The batch rectifier consists of a pot (or reboiler), rectifying column, a condenser, some means of splitting off a portion of the condensed vapour (distillate) as reflux, and one or more receivers. The pot is filled with liquid mixture and heated. Vapour flows upwards in the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Distillation
Steam distillation is a separation process that consists of distilling water together with other volatile and non-volatile components. The steam from the boiling water carries the vapor of the volatiles to a condenser; both are cooled and return to the liquid or solid state, while the non-volatile residues remain behind in the boiling container. If, as is usually the case, the volatiles are not miscible with water, they will spontaneously form a distinct phase after condensation, allowing them to be separated by decantation or with a separatory funnel. Steam distillation can be used when the boiling point of the substance to be extracted is higher than that of water, and the starting material cannot be heated to that temperature because of decomposition or other unwanted reactions. It may also be useful when the amount of the desired substance is small compared to that of the non-volatile residues. It is often used to separate volatile essential oils from plant material. f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heteroazeotrope
A heteroazeotrope is an azeotrope where the vapour phase coexists with two liquid phases. Sketch of a T-x/y equilibrium curve of a typical heteroazeotropic mixture Examples of heteroazeotropes *Benzene - Water NBP 69.2 °C *Dichloromethane - Water NBP 38.5 °C * n-Butanol - Water NBP 93.5 °C * Toluene - Water NBP 82 °C Continuous heteroazeotropic distillation Heterogeneous distillation means that during the distillation the liquid phase of the mixture is immiscible. In this case on the plates can be two liquid phases and the top vapour condensate splits in two liquid phases, which can be separated in a decanter. The simplest case of continuous heteroazeotropic distillation is the separation of a binary heterogeneous azeotropic mixture. In this case the system contains two columns and a decanter. The fresh feed (A-B) is added into the first column. (The feed may also be added into the decanter directly or into the second column depending on the composition of the mixture). Fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloromethane
Dichloromethane (DCM, methylene chloride, or methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.Rossberg, M. ''et al.'' (2006) "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. . Occurrence Natural sources of dichloromethane include oceanic sources, macroalgae, wetlands, and volcanoes. However, the majority of dichloromethane in the environment is the result of industrial emissions. Production DCM is produced by treating either chloromethane or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and chloromethane undergo a series of reactions producing progressively more chlorinated products. In this way, an estimated 400,000 tons were produced in the US, Europe, and Japan in 199 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Butanol
1-Butanol, also known as butan-1-ol or ''n''-butanol, is a Alcohol (chemistry), primary alcohol with the chemical formula C4H9OH and a linear structure. Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol, ''tert''-butanol. The unmodified term butanol usually refers to the straight chain isomer. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and other saccharides and is present in many foods and drinks... It is also a permitted artificial flavorant in the United States, used in butter, cream, fruit, rum, whiskey, ice cream and ices, candy, baked goods, and cordials. It is also used in a wide range of consumer products. The largest use of 1-butanol is as an industrial intermediate, particularly for the manufacture of butyl acetate (itself an artificial flavorant and industrial solvent). It is a petrochemical derived from propylene. Estimated production figures for 1997 are: United States 784,000 tonnes; Western Europe 575,000&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC nomenclature of organic chemistry, IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conti Hetero 1
Conti is an Italian surname. Geographical distribution As of 2014, 63.5% of all known bearers of the surname ''Conti'' were residents of Italy (frequency 1:756), 11.8% of the United States (1:24,071), 9.2% of Brazil (1:17,439), 6.3% of Argentina (1:5,300), 2.5% of France (1:21,201) and 1.3% of the Philippines (1:58,961). In Italy, the frequency of the surname was higher than national average (1:756) in the following regions: # Tuscany (1:360) # Umbria (1:363) # Marche (1:370) # Lazio (1:412) # Emilia-Romagna (1:478) # Lombardy (1:531) # Sicily (1:624) # Liguria (1:628) In Argentina, the frequency of the surname was higher than national average (1:5,300) in the following provinces: # Santa Fe Province (1:3,222) # Córdoba Province (1:3,292) # Buenos Aires (1:4,110) # Mendoza Province (1:4,201) # Buenos Aires Province (1:4,408) # La Pampa Province (1:4,731) People * The historical Conti di Segni, family ** Andrea dei Conti (1240–1302), Italian Roman Catholic priest ** Giovanni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |