|
Hapten
Haptens (derived from the Greek ''haptein'', meaning “to fasten”) are small molecules that elicit an immune response only when attached to a large carrier such as a protein; the carrier may be one that also does not elicit an immune response by itself. The mechanisms of absence of immune response may vary and involve complex immunological interactions, but can include absent or insufficient co-stimulatory signals from antigen-presenting cells. Haptens have been used to study allergic contact dermatitis (ACD) and the mechanisms of inflammatory bowel disease (IBD) to induce autoimmune-like responses. The concept of haptens emerged from the work of Austrian immunologist Karl Landsteiner, who also pioneered the use of synthetic haptens to study immunochemical phenomena. Immune reaction on a hapten-carrier adduct Haptens applied on skin, when conjugate with a carrier, could induce contact hypersensitivity, which is a type IV delayed hypersensitivity reaction mediated by T cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a form of contact dermatitis that is the manifestation of an allergic response caused by contact with a substance; the other type being irritant contact dermatitis (ICD). Although less common than ICD, ACD is accepted to be the most prevalent form of immunotoxicity found in humans. By its allergic nature, this form of contact dermatitis is a hypersensitive reaction that is atypical within the population. The mechanisms by which these reactions occur are complex, with many levels of fine control. Their immunology centres on the interaction of immunoregulatory cytokines and discrete subpopulations of T lymphocytes. Signs and symptoms The symptoms of allergic contact dermatitis are very similar to the ones caused by irritant contact dermatitis, which makes the first even harder to diagnose. The first sign of allergic contact dermatitis is the presence of the rash or skin lesion at the site of exposure. Depending on the type of allergen causi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Allergy
Nickel allergy is any of several allergy, allergic conditions provoked by exposure to the chemical element nickel. Nickel allergy often takes the form of nickel allergic contact dermatitis (Ni-ACD), a form of allergic contact dermatitis (ACD). Ni-ACD typically causes a rash that is red and itchy and that may be bumpy or scaly. The main treatment for it is avoiding contact with nickel-releasing metals, such as inexpensive jewelry. Another form of nickel allergy is a systemic disease, systemic form: systemic nickel allergy syndrome (SNAS) can mimic some of the symptoms of irritable bowel syndrome (IBS) and also has a dermatologic component. Signs and symptoms The most common sign of nickel allergy is inflammation of the skin at an area that comes into regular contact with nickel. This often takes the form of a Erythema, reddened patch of skin with raised bumps (papules) or small blisters (Vesicle (dermatology), vesicles), and edema. People with chronic dermatitis tend to have dry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
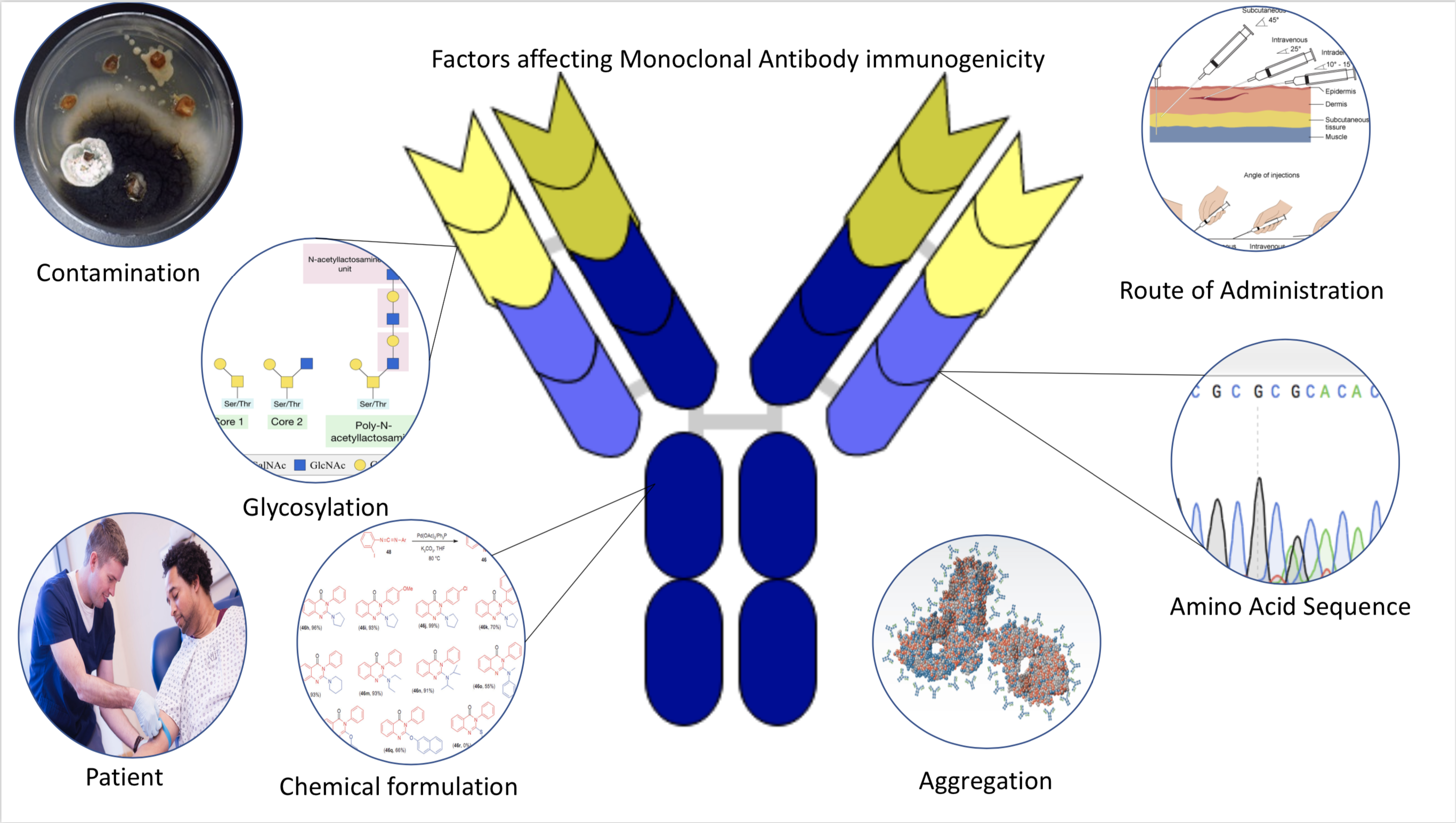
Immunogenicity
Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted: * Wanted immunogenicity typically relates to vaccines, where the injection of an antigen (the vaccine) provokes an immune response against the pathogen, protecting the organism from future exposure. Immunogenicity is a central aspect of vaccine development. * Unwanted immunogenicity is an immune response by an organism against a therapeutic antigen. This reaction leads to production of anti-drug-antibodies (ADAs), inactivating the therapeutic effects of the treatment and potentially inducing adverse effects. A challenge in biotherapy is predicting the immunogenic potential of novel protein therapeutics. For example, immunogenicity data from high-income countries are not always transferable to low-income and middle-income countries. Another challenge is considering how the immunogenicity of vaccines changes wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urushiol-induced Contact Dermatitis
Urushiol-induced contact dermatitis (also called Toxicodendron dermatitis or Rhus dermatitis) is a type of allergic contact dermatitis caused by the oil urushiol found in various plants, most notably sumac family species of the genus ''Toxicodendron'': poison ivy, Toxicodendron diversilobum, poison oak, poison sumac, and the Toxicodendron vernicifluum, Chinese lacquer tree. The name is derived from the Japanese word for the sap of the Chinese lacquer tree, ''urushi''. Other plants in the Anacardiaceae, sumac family (including mango, pistachio, the Burmese lacquer tree, the India marking nut tree, and the cashew) also contain urushiol, as do unrelated plants such as ''Ginkgo biloba.'' As is the case with all contact dermatitis, urushiol-induced allergy, allergic rashes are a Type IV hypersensitivity, Type IV hypersensitivity reaction, also known as delayed-type hypersensitivity. Symptoms include itching, inflammation, oozing, and, in severe cases, a burning sensation. The American ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urushiol
Urushiol is an oily mixture of organic compounds with Allergic contact dermatitis, allergenic properties found in plants of the Family (biology), family Anacardiaceae, especially ''Toxicodendron'' ''spp.'' (e.g., poison oak, Toxicodendron vernicifluum, Chinese lacquer tree, Toxicodendron radicans, poison ivy, Toxicodendron vernix, poison sumac), Comocladia, ''Comocladia spp.'' (maidenplums), Metopium, ''Metopium spp''. (poisonwood), and also in parts of the Mangifera indica, mango tree and the fruit of the cashew tree. In most individuals, urushiol causes an allergic skin rash on contact, known as urushiol-induced contact dermatitis. The name urushiol is derived from the Japanese language, Japanese word for the lacquer tree, . The oxidation and polymerization of urushiol in the tree's sap in the presence of moisture allows it to form a hard lacquer, which is used to produce traditional Chinese, Korean, and Japanese lacquerware. History Although urushiol-containing lacquers a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small Molecule
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; the terms are equivalent in the literature. Larger structures such as nucleic acids and proteins, and many polysaccharides are not small molecules, although their constituent monomers (ribo- or deoxyribonucleotides, amino acids, and monosaccharides, respectively) are often considered small molecules. Small molecules may be used as research tools to probe biological function as well as leads in the development of new therapeutic agents. Some can inhibit a specific function of a protein or disrupt protein–protein interactions. Pharmacology usually restricts the term "small molecule" to molecules that bind specific biological macromolecules and act as an effector, altering the activity or function of the target. Small molecules can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Aminobenzoic Acid
3-Aminobenzoic acid (also known as ''meta''-aminobenzoic acid or MABA) is an organic compound with the molecular formula H2NC6H4CO2H. MABA is a white solid, although commercial samples are often colored. It is only slightly soluble in water. It is soluble in acetone, boiling water, hot alcohol, hot chloroform and ether. It consists of a benzene ring substituted with an amino group and a carboxylic acid. It is prepared by reduction of 3-nitrobenzoic acid. It is used to prepare some dyes. See also * Aminomethylbenzoic acid * Anthranilic acid (2-aminobenzoic acid) * 4-Aminobenzoic acid * Arene substitution pattern * Non-proteinogenic amino acids In biochemistry, non-coded or non-proteinogenic amino acids are distinct from the 22 proteinogenic amino acids (21 in eukaryotesplus formylmethionine in eukaryotes with prokaryote organelles like mitochondria), which are naturally encoded in the ... References {{DEFAULTSORT:Aminobenzoic acid, 3- 3-Aminophenyl compounds Benzoic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthranilic Acid
Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste. The molecule consists of a benzene ring, ''ortho''-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion 6H4(NH2)(CO2)sup>−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition. Structure Although not usually referred to as such, it is an amino acid. Solid anthranilic acid typically consists of both the amino-carboxylic acid and the zwitterionic ammonium carboxylate forms, and has a monoclinic crystal structure with space group P21. It is triboluminescent. Above , it converts to an orthorhombic form with spa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dalton (unit)
The dalton or unified atomic mass unit (symbols: Da or u, respectively) is a unit of mass defined as of the mass of an Bound state, unbound neutral atom of carbon-12 in its nuclear and electronic ground state and invariant mass, at rest. It is a Non-SI units mentioned in the SI, non-SI unit accepted for use with SI. The word "unified" emphasizes that the definition was accepted by both IUPAP and IUPAC. The atomic mass constant, denoted , is defined identically. Expressed in terms of , the atomic mass of carbon-12: . Its value in International System of Units, SI units is an experimentally determined quantity. The 2022 CODATA recommended value of the atomic mass constant expressed in the SI base unit kilogram is:This value serves as a Conversion of units, conversion factor of mass from daltons to kilograms, which can easily be converted to Gram, grams and other metric units of mass. The 2019 revision of the SI redefined the kilogram by fixing the value of the Planck constant (), i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |