|
Griseofulvin
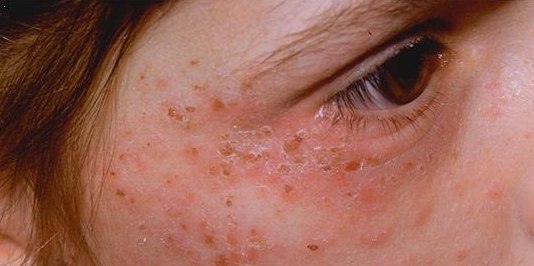
Griseofulvin is an antifungal medication used to treat a number of types of dermatophytoses (ringworm). This includes fungal infections of the nails and scalp, as well as the skin when antifungal creams have not worked. It is taken by mouth. Common side effects include allergic reactions, nausea, diarrhea, headache, trouble sleeping, and feeling tired. It is not recommended in people with liver failure or porphyria. Use during or in the months before pregnancy may result in harm to the baby. Griseofulvin works by interfering with fungal mitosis. Griseofulvin was discovered in 1939 from the soil fungus '' Penicillium griseofulvum''. It is on the World Health Organization's List of Essential Medicines. Medical uses Griseofulvin is used orally only for dermatophytosis. It is ineffective topically. It is reserved for cases in which topical treatment with creams is ineffective. Terbinafine given for 2 to 4 weeks is at least as effective as griseofulvin given for 6 to 8 weeks f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dermatophyte
Dermatophyte (from Greek '' derma'' "skin" ( GEN ''dermatos'') and ''phyton'' "plant") is a common label for a group of fungus of '' Arthrodermataceae'' that commonly causes skin disease in animals and humans. Traditionally, these anamorphic (asexual or imperfect fungi) mold genera are: '' Microsporum'', '' Epidermophyton'' and ''Trichophyton''. There are about 40 species in these three genera. Species capable of reproducing sexually belong in the teleomorphic genus Arthroderma, of the Ascomycota (see Teleomorph, anamorph and holomorph for more information on this type of fungal life cycle). As of 2019 a total of nine genera are identified and new phylogenetic taxonomy has been proposed. Dermatophytes cause infections of the skin, hair, and nails, obtaining nutrients from keratinized material. The organisms colonize the keratin tissues causing inflammation as the host responds to metabolic byproducts. Colonies of dermatophytes are usually restricted to the nonliving cornifie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyria
Porphyria ( or ) is a group of disorders in which substances called porphyrins build up in the body, adversely affecting the skin or nervous system. The types that affect the nervous system are also known as Porphyria#Acute porphyrias, acute porphyria, as symptoms are rapid in onset and short in duration. Symptoms of an attack include abdominal pain, chest pain, vomiting, confusion, constipation, fever, hypertension, high blood pressure, and tachycardia, high heart rate. The attacks usually last for days to weeks. Complications may include paralysis, hyponatraemia, low blood sodium levels, and seizures. Attacks may be triggered by Alcohol (drug), alcohol, smoking, hormonal changes, fasting, stress, or certain medications. If the skin is affected, blisters or itching may occur with sunlight exposure. Most types of porphyria are inherited from one or both of a person's parents and are due to a mutation in one of the genes that make heme. They may be inherited in an autosomal dom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. It was discovered and named by Hideo Mōri in 1968. Microtubules function in many essential cellular processes, including mitosis. Discovery and development of tubulin inhibitors, Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division. #Eukaryotic, In eukaryotes, there are six members of the tubulin superfamily, although not all are present in all species.Turk E, Wills AA, Kwon T, Sedzinski J, Wallingford JB, Stearns "Zeta-Tubulin Is a Member of a Conserved Tubulin Module and Is a Component of the Centriolar Basal Foot in Multiciliated Cells"Current Biology (2015) 25:2177-2183. Both α and β tubulins have a mass of around 50 kDa and are thus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration whereby a substance is taken through the Human mouth, mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications. Oral administration can be easier and less painful than other routes of administration, such as Injection (medicine), injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth". The expression is used in medicine to describe a treatment that is taken orally (but not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terbinafine
Terbinafine, sold under the brand name Lamisil among others, is an antifungal medication used to treat pityriasis versicolor, onychomycosis, fungal nail infections, and ringworm including jock itch and athlete's foot. It is either oral administration, taken by mouth or applied to the skin as a cream or ointment. Common side effects when taken by mouth include nausea, diarrhea, headache, cough, rash, and elevated liver enzymes. Severe side effects include liver problems and allergic reactions. Liver injury is, however, unusual. Oral use during pregnancy is not typically recommended. The cream and ointment may result in itchiness but are generally well tolerated. Terbinafine is in the allylamines family of medications. It works by decreasing the ability of fungi to synthesize ergosterol. It appears to result in fungicide, fungal cell death. Terbinafine was discovered in 1991. It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide Phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a Cofactor (biochemistry), cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the redox, reduced form, whereas NADP is the redox, oxidized form. NADP is used by all forms of cellular life. NADP is essential for life because it is needed for cellular respiration. NADP differs from NAD+, NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine Moiety (chemistry), moiety. This extra phosphate is added by NAD+ kinase, NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+, NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase, NAD+ kinase adding the extra phosphate g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceuti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-Adenosyl Methionine
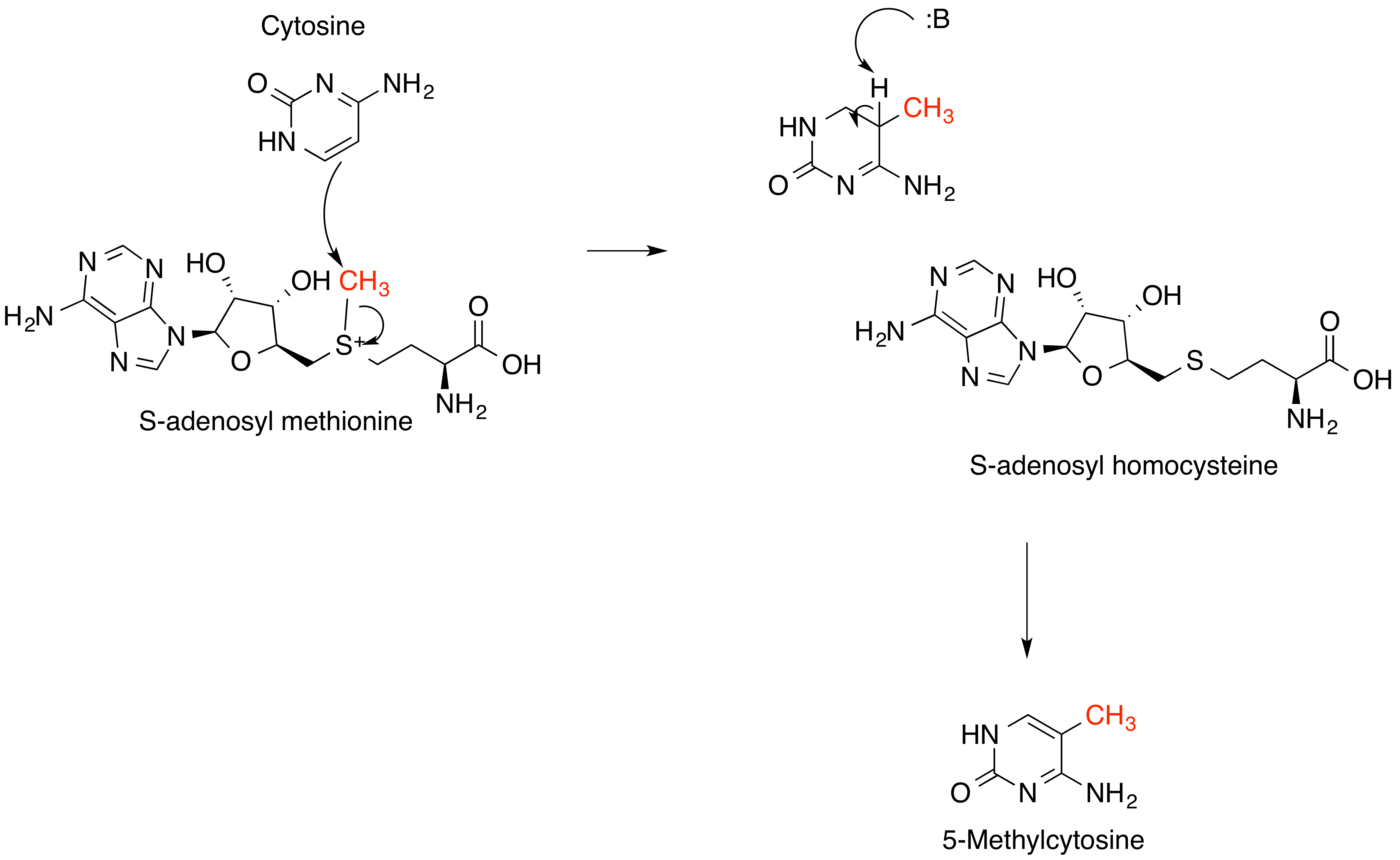
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylated
Methylation, in the chemical sciences, is the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and biology. In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. ''In vitro'' methylation of tissue samples is also a way to reduce some histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals and can regulate gene expression, RNA processing, and protein function. It is a key process underlying epigenetics. Sources of methyl groups include S-methylmethionine, methyl folate, methyl B12. Me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and rose-like odor that is soluble in organic solvents. Benzophenone is the simplest diaromatic ketone. It is a widely used building block in organic chemistry, being the parent diarylketone. History Carl Graebe of the University of Königsberg, in an early literature report from 1874, described working with benzophenone. Uses Benzophenone can be used as a photo initiator in ultraviolet (UV)-curing applications such as inks, imaging, and clear coatings in the printing industry. Benzophenone prevents UV light from damaging scents and colors in products such as perfumes and soaps. Benzophenone can also be added to plastic packaging as a UV blocker to prevent photo-degradation of the packaging polymers or its contents. Its use allows manufactur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction equation is as follows (where the Rs can be H) Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or aldol (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, the first step is formally an addition reaction rather than a condensation reaction bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base. The reaction produces a β-keto ester or a β- diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. The reaction has often been displaced by diketene-based chemistry, which affords acetoacetic esters. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |