|
Glycidyl Methacrylate
Glycidyl methacrylate (GMA) is an ester of methacrylic acid and glycidol. Containing both an epoxide and an acrylate group, the molecule is bifunctional. It is a common monomer A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization. Classification Chemis ... used in the production of epoxy resins. While typical home epoxies contain diglycidyl ether of bisphenol A (DGEBA), glycidyl methacrylate is instead used to provide epoxy functionalization to polyolefins and other acrylate resins. Glycidyl methacrylate is produced by several companies worldwide, including Dow Chemical. It is used to prepare a range of composites.{{cite journal , doi=10.1038/nmat1890, title=Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration , year=2007 , last1=Wang , first1=Dong-An , last2=Varghese , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functionalized Polyolefins
Functionalized polyolefins are Polyolefin, olefin polymers with polar and nonpolar Functional group, functionalities attached onto the polymer backbone. There has been an increased interest in functionalizing polyolefins due to their increased usage in everyday life. Polyolefins are virtually ubiquitous in everyday life, from consumer food packaging to biomedical applications; therefore, efforts must be made to study Catalysis, catalytic pathways towards the attachment of various functional groups onto polyolefins in order to affect the material's physical properties. Based on the polyolefin structure, functionalized polyolefin can be categorized into four main groups: randomly functionalized polyolefins, end-functionalized polyolefins, block polyolefins, and graft polyolefins. Randomly functionalized polyolefin Randomly functionalized polyolefins have differing types, location, and amount of functionality on the polyolefin backbone. Randomly functionalized polyolefins can be s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxides
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom Ring (chemistry), ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly Reactivity (chemistry), reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often Volatility (chemistry), volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomers
A monomer ( ; ''wikt:mono-, mono-'', "one" + ''wikt:-mer, -mer'', "part") is a molecule that can chemical reaction, react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization. Classification Chemistry classifies monomers by type, and two broad classes based on the type of polymer they form. By type: * natural vs synthetic, e.g. glycine vs caprolactam, respectively * polar vs nonpolar, e.g. vinyl acetate vs ethylene, respectively * cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively By type of polymer they form: * those that participate in condensation polymerization * those that participate in addition polymerization Differing stoichiometry causes each class to create its respective form of polymer. : The polymerization of one kind of monomer gives a polymer#Monomers and repeat units, homopolymer. Many polymers are copolymers, meaning that they are derived from two diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methacrylate
Methacrylates are derivatives of methacrylic acid. These derivatives are mainly used to make poly(methyl methacrylate) and related polymers. *Monomers ** Methyl methacrylate ** Ethyl methacrylate ** Butyl methacrylate ** Hydroxyethyl methacrylate ** Glycidyl methacrylate Glycidyl methacrylate (GMA) is an ester of methacrylic acid and glycidol. Containing both an epoxide and an acrylate group, the molecule is bifunctional. It is a common monomer A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecu ... {{set index Carboxylate anions Monomers Methacrylate esters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
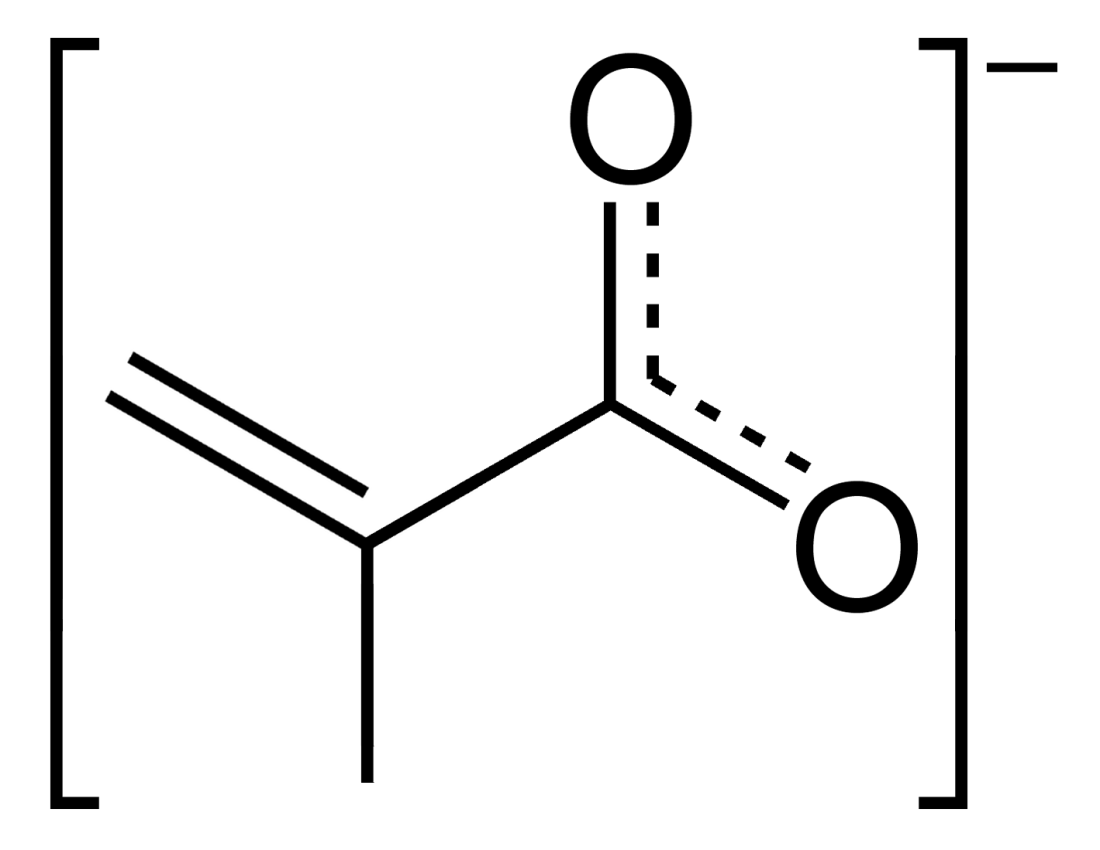
Acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion . Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain vinyl groups. These compounds are of interest because they are bifunctional: the vinyl group is susceptible to polymerization and the carboxylate group carries myriad functionalities. Monomers Acrylates are defined by the formula , where R can be many groups: * Acrylic acid * Methyl acrylate * Ethyl acrylate * 2-Chloroethyl vinyl ether * 2-Ethylhexyl acrylate * Butyl acrylate * Trimethylolpropane triacrylate (TMPTA) The versatility of the resulting polymers is owed to the range of R groups. File:Acrylate-anion.svg, The acrylate anion File:Trimethylolpropane triacrylate.svg, Trimethylolpropane triacrylate (TMPTA), a trifunctional acrylate ester File:Methylacrylat.svg, Methyl acrylate, an acrylic ester File:Hexandioldiacrylat.svg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acrylate Polymer
An acrylate polymer (also known as acrylic or polyacrylate) is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity. Acrylate polymer is commonly used in cosmetics, such as nail polish, as an adhesive. History The first synthesis of acrylic polymer was reported by G. W. A. Kahlbaum in 1880. Acrylic elastomers Acrylic elastomer is a general term for a type of synthetic rubber whose primary component is acrylic acid alkylester ( ethyl or butyl ester). Acrylic elastomer possesses characteristics of heat and oil resistance, with the ability to withstand temperatures of 170–180 °C. It is used primarily for producing oil seals and packaging related to automobiles. Acrylic elastomer can generally be characterized as one of two types. "Old" types include ACM ( copolymer of acrylic acid ester and 2-chloroethyl vinyl ether) containing chlorine and ANM (copolymer of acrylic acid e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dow Chemical
The Dow Chemical Company is an American multinational corporation headquartered in Midland, Michigan, United States. The company was among the three largest chemical producers in the world in 2021. It is the operating subsidiary of Dow Inc., a publicly traded holding company incorporated under Delaware law. With a presence in around 160 countries, it employs about 36,000 people worldwide. Dow has been called the "chemical companies' chemical company", as its sales are to other industries rather than directly to end-use consumers. Dow is a member of the American Chemistry Council. In 2015, Dow and fellow chemical company DuPont agreed to a corporate reorganization involving the merger of Dow and DuPont followed by a separation into three different entities. The plan commenced in 2017, when Dow and DuPont merged to form DowDuPont, and was finalized in April 2019, when the materials science division was spun off from DowDuPont and took the name of the Dow Chemical Company. Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diglycidyl Ether Of Bisphenol A
Bisphenol A diglycidyl ether (commonly abbreviated BADGE or DGEBA) is an organic compound and is a liquid epoxy resin. The compound is a colorless viscous liquid (commercial samples can appear pale straw-coloured). It is a key component of many epoxy resin formulations. Addition of further Bisphenol A and a catalyst and heat can produce Bisphenol A glycidyl ether epoxy resins of higher molecular weight that are solid. Preparation and reactions It is prepared by O-alkylation of bisphenol A with epichlorohydrin. This reaction mainly affords bisphenol A diglycidyl ether, as well as some oligomer. The degree of polymerization may be as low as 0.1. The epoxide content of such epoxy resins is of interest. This parameter is commonly expressed as the epoxide number, which is the number of epoxide equivalents in 1 kg of resin (Eq./kg), or as the equivalent weight, which is the weight in grams of resin containing 1 mole equivalent of epoxide (g/mol). Since unsymmetrical epoxides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methacrylic Acid
Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)CO2H. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA). Production In the most common route, methacrylic acid is prepared from acetone cyanohydrin, which is converted to methacrylamide sulfate using sulfuric acid. This derivative in turn is hydrolyzed to methacrylic acid, or esterified to methyl methacrylate in one step. Another route to methacrylic acid starts with isobutylene, which obtainable by dehydration of ''tert''-butanol. Isobutylene is oxidized sequentially to methacrolein and then methacrylic acid. Methacrolein for this purpose can also be obtained from formaldehyde and ethylene. Yet a third route involves the dehy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxy
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy Resin, resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called ''epoxy''. The IUPAC name for an epoxide group is an oxirane. Epoxy resins may be reacted (cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols, alcohols and thiols (sometimes called mercaptans). These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as Curing (chemistry), curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance. Epoxy has a wide range of application ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization. Classification Chemistry classifies monomers by type, and two broad classes based on the type of polymer they form. By type: * natural vs synthetic, e.g. glycine vs caprolactam, respectively * polar vs nonpolar, e.g. vinyl acetate vs ethylene, respectively * cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively By type of polymer they form: * those that participate in condensation polymerization * those that participate in addition polymerization Differing stoichiometry causes each class to create its respective form of polymer. : The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |