|
Glycerolysis
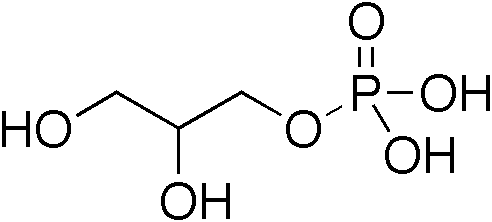
In organic chemistry glycerolysis refers to any process in which chemical bonds are broken via a reaction with glycerol. The term refers almost exclusively to the transesterification reaction of glycerol with triglycerides (fats/oils) to form mixtures of monoglycerides and diglycerides. These find a variety of uses; as food emulsifiers (e.g. E471), 'low fat' cooking oils (e.g. diacylglycerol oil) and surfactants (such as monolaurin). The transesterification process gives a complex mixture of products, however not all of these are of equivalent use. This has led to the development of optimized processes able to produce better defined products; in particular by using enzymes, reactions in supercritical carbon dioxide and flow chemistry. The production of diglycerides (often called diacylglycerols or DAGs) have been investigated extensively due to their use in foods, with total annual sales of approximately US$200 million in Japan since its introduction in the late 1990s until 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diglyceride
A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil (particularly 1,3-DAG) has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009. Production Diglycerides are a minor component of many seed oils and are normally present at ~1–6%; or in the case of cottonseed oil as much as 10%. Industrial production is primarily achieved by a glycerolysis reaction between triglycerides and glycerol. The raw materials for this may be either vegetable oil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
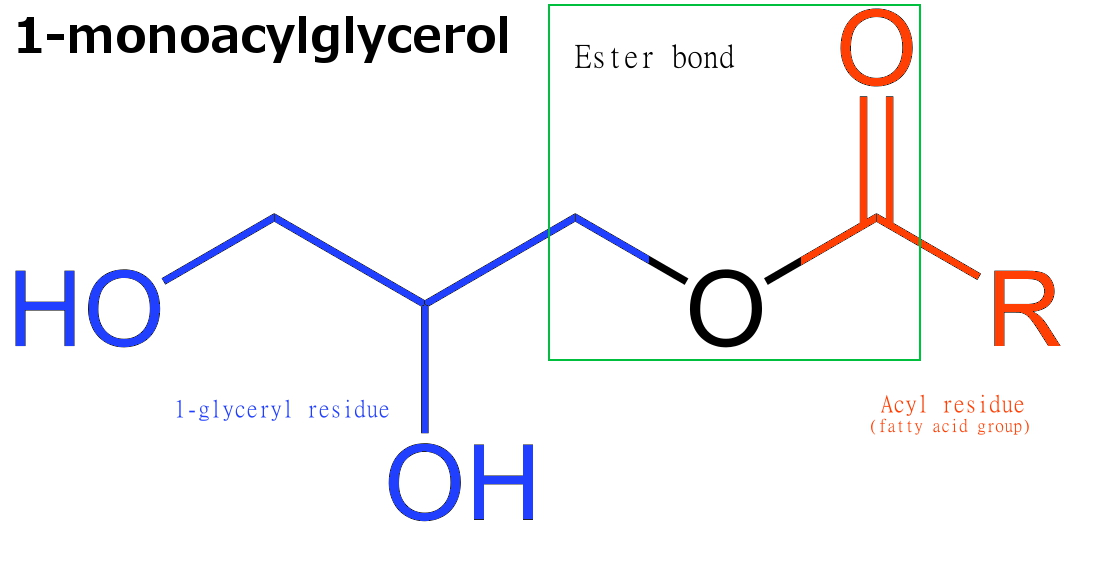
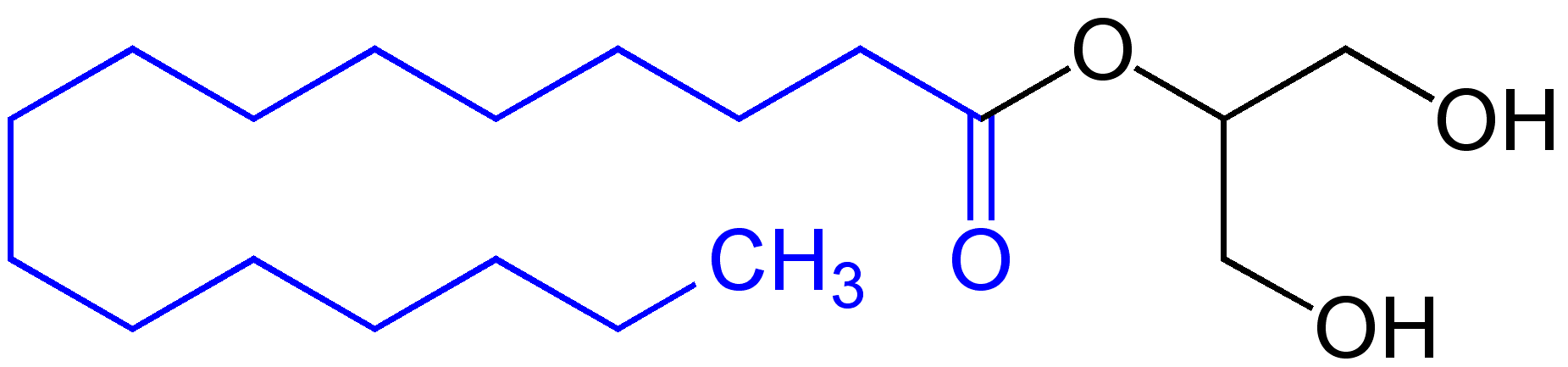
Monoglyceride
Monoglycerides (also: acylglycerols or monoacylglycerols) are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol. Synthesis Monoglycerides are produced both biologically and industrially. They are naturally present at very low levels (0.1-0.2%) in some seed oils such as olive oil, rapeseed oil and cottonseed oil. They are biosynthesized by the enzymatic hydrolysis of triglycerides by lipoprotein lipase and the enzymatic hydrolysis of diglycerides by diacylglycerol lipase; or as an intermediate in the alkanoylation of glycerol to form fats. Several monoglycerides are pharmacologically active (e.g. 2-oleoylglycerol, 2-arachidonoylglycerol). Industrial pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mono- And Diglycerides Of Fatty Acids
Mono- and diglycerides of fatty acids ( E471) are a naturally occurring class of food additive composed of diglycerides and monoglycerides used as an emulsifier in foods such as infant formula, fresh pasta, jams and jellies, chocolate, creams, baked goods, and more. It is also used as a fruit coating agent. This mixture is also sometimes referred to as partial glycerides. Synthesis Monoglycerides and diglycerides are types of glycerides both naturally present in food fats, including various seed oils; however, their concentration is usually low and industrial production is primarily achieved by a glycerolysis reaction between triglycerides (fats/oils) and glycerol, followed by purification via solvent-free molecular distillation. The raw materials of mono- and diglycerides may be either vegetable or animal fats and oils. Dietary aspects E471 is mainly produced from vegetable oils (such as soybean, grapeseed, canola, sunflower, cottonseed, coconut, and palm oil) and plant p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymes
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include catalytic RNA molecules, also called ribozymes. They are sometimes described as a ''type'' of enzyme rather than being ''like'' an enzyme, but even in the d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylate Esters
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge. Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,.... Carboxylate esters have the general formula (also written as ), where R and R′ are organic groups. Synthesis Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such as sodium hydroxide or sodium bicarbonate. : Resonance stabilization of the carboxylate ion Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between the two electronegativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transesterification
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of enzymes, particularly lipases (one example is the lipase E.C.3.1.1.3). If the alcohol produced by the reaction can be separated from the reactants by distillation this will drive the equilibrium toward the products. This means that esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol. Mechanism In the transesterification mechanism, the carbonyl carbon of the starting ester reacts to give a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saponification
Saponification is a process of cleaving esters into carboxylate salts and Alcohol (chemistry), alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. It is an important type of alkaline hydrolysis. When the carboxylate is long chain, its salt is called a soap. The saponification of ethyl acetate gives sodium acetate and ethanol: : Saponification of fats Vegetable oils and animal fats are the traditional materials that are saponified. These greasy materials, triesters called triglycerides, are usually mixtures derived from diverse fatty acids. In the traditional saponification, the triglyceride is treated with lye, which cleaves the ester bonds, releasing fatty acid salts (soaps) and glycerol. In one simplified version, the saponification of stearin gives sodium stearate. : This process is the main industrial method for producing glycerol (). Some soap-makers leave the glycerol in the soap. Others precipitation (chemistry), precipitate t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flow Chemistry
In flow chemistry, also called reactor engineering, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a reactor, and where tubes join one another, the fluids contact one another. If these fluids are reactive, a reaction takes place. Flow chemistry is a well-established technique for use at a large scale when manufacturing large quantities of a given material. However, the term has only been coined recently for its application on a laboratory scale by chemists and describes small pilot plants, and lab-scale continuous plants. Often, microreactors are used. Batch vs. flow Comparing parameter definitions in Batch vs Flow *Reaction stoichiometry: In batch production this is defined by the concentration of chemical reagents and their volumetric ratio. In flow this is defined by the concentration of reagents and the ratio of their flow rate. *Residence time: In batch production this is determined ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercritical Carbon Dioxide
Supercritical carbon dioxide (s) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure. Carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP), or as a solid called dry ice when cooled and/or pressurised sufficiently. If the temperature and pressure are both increased from STP to be at or above the critical point for carbon dioxide, it can adopt properties midway between a gas and a liquid. More specifically, it behaves as a supercritical fluid above its critical temperature () and critical pressure (), expanding to fill its container like a gas but with a density like that of a liquid. Supercritical is becoming an important commercial and industrial solvent due to its role in chemical extraction, in addition to its relatively low toxicity and environmental impact. The relatively low temperature of the process and the stability of also allows compounds to be extracted with little ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a Blend word, blend of "surface-active agent", coined in 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt. Surfactants are among the most widespread and commercially important chemicals. Private households as well as many industries use them in large quantities as detergent, detergents and cleaning agents, but also for example as emulsion#Emulsifiers, emulsifiers, wetting agents, foaming agents, Antistatic agent, antistatic additives, or dispersants. Surfactants occur naturally in traditional plant-based detergents, e.g. Aesculus, horse chestnuts or Sapindus, soap nuts; they can also be found in the secretions of some caterpillars. Today one of the most commonly used anionic surfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |