|
Glushinskite
Glushinskite is an oxalate mineral and the mineral form of magnesium oxalate.Page ''Glushinskite: Mineral information, data and localities'' on Occurrences of glushinskite are commonly associated with the growth of lichen on serpentinite Serpentinite is a metamorphic rock composed predominantly of serpentine group minerals formed by serpentinization of mafic or ultramafic rocks. The ancient origin of the name is uncertain; it may be from the similarity of its texture or color .... References External links Oxalate minerals Magnesium minerals Dihydrate minerals Monoclinic minerals Minerals described in 1980 {{mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxalate
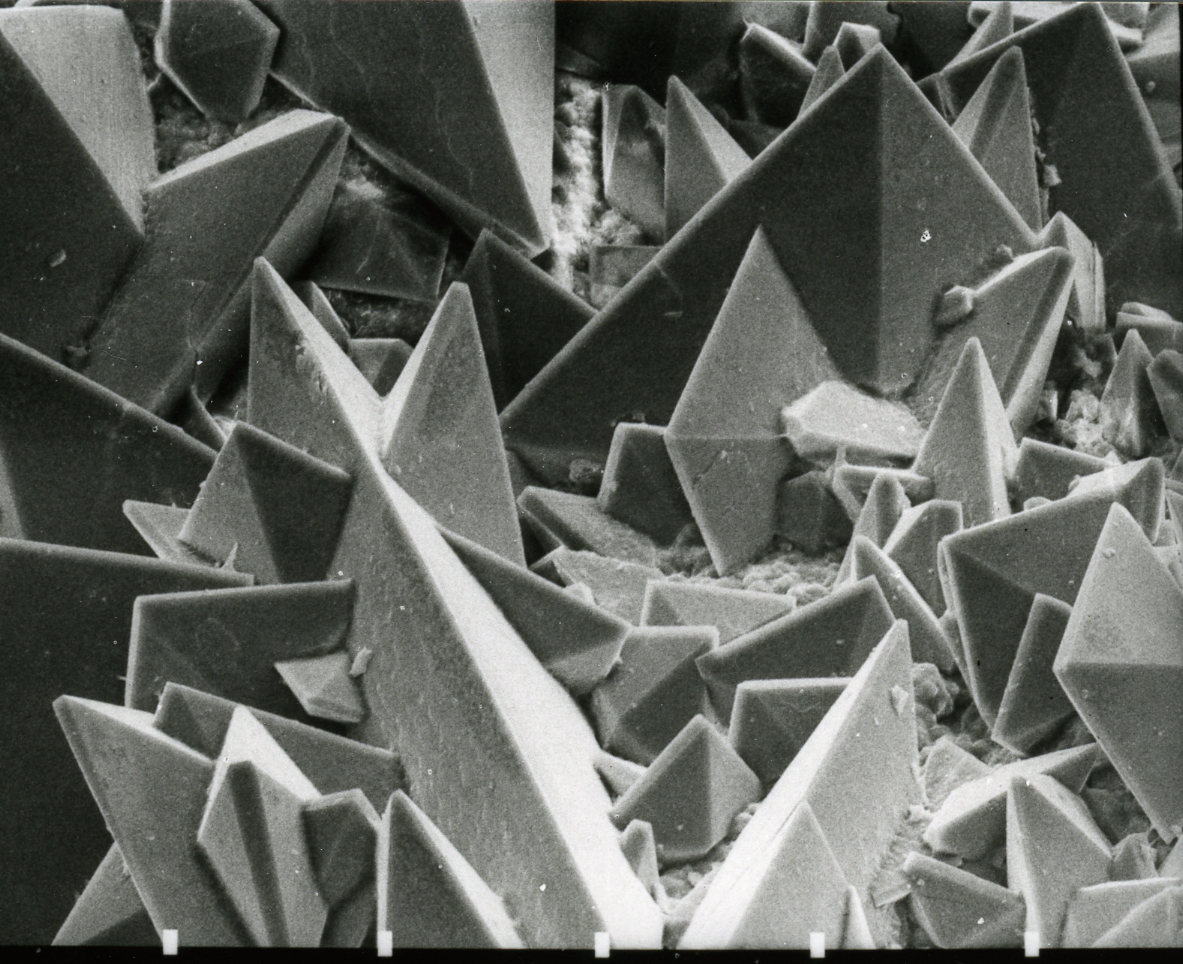
Magnesium oxalate is an organic compound comprising a magnesium cation with a 2+ charge bonded to an oxalate anion. It has the chemical formula MgCO. Magnesium oxalate is a white solid that comes in two forms: an anhydrous form and a dihydrate form where two water molecules are complexed with the structure. Both forms are practically insoluble in water and are insoluble in organic solutions. Natural occurrence Magnesium oxalate has been found naturally near Mill of Johnston, which is located close to Insch in northeast Scotland. This naturally occurring magnesium oxalate is called glushinskite and occurs at the lichen/rock interface on serpentinite as a creamy white layer mixed in with the hyphae of the lichen fungus. A scanning electron micrograph of samples taken showed that the crystals had a pyramidal structure with both curved and striated faces. The size of these crystals ranged from 2 to 5 μm. Synthesis and reactions Magnesium oxalate can by synthesized by co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalate Mineral
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as dimethyl oxalate (). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate. Relationship to oxalic acid The dissociation of protons from oxalic acid proceeds in a determined order; as for other polyprotic acids, loss of a single proton results in the monovalent hydrogenoxalate anion . A salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant ( ''K''a) for loss of the first proton is ( p''K''a = 1.27). The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of (p''K''a = 4.28). These values imply, in solutions with neutral pH, no oxalic acid and only tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism (geometry), prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. The length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal class ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalate Mineral
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as dimethyl oxalate (). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate. Relationship to oxalic acid The dissociation of protons from oxalic acid proceeds in a determined order; as for other polyprotic acids, loss of a single proton results in the monovalent hydrogenoxalate anion . A salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant ( ''K''a) for loss of the first proton is ( p''K''a = 1.27). The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of (p''K''a = 4.28). These values imply, in solutions with neutral pH, no oxalic acid and only tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hudson Institute Of Mineralogy
Mindat.org is a non-commercial interactive online database covering minerals around the world. Originally created by Jolyon Ralph as a private project in 1993, it was launched as a community-editable website in October 2000. it is operated by the Hudson Institute of Mineralogy. History Mindat was started in 1993 as a personal database project by Jolyon Ralph. He then developed further versions as a Microsoft Windows application before launching a community-editable database website on 10 October 2000. After further development taking to the Internet stage, Mindat.org became an outreach program of the Hudson Institute of Mineralogy, a 501(c)(3) not-for-profit educational foundation incorporated in the New York state, state of New York. To address the increasing open data needs from individual researchers and organizations, Mindat.org has started to build and maintain an open data API for data query and access, and the efforts have received support from the National Science ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lichen
A lichen ( , ) is a hybrid colony (biology), colony of algae or cyanobacteria living symbiotically among hypha, filaments of multiple fungus species, along with yeasts and bacteria embedded in the cortex or "skin", in a mutualism (biology), mutualistic relationship.Introduction to Lichens – An Alliance between Kingdoms . University of California Museum of Paleontology. . Lichens are the lifeform that first brought the term symbiosis (as ''Symbiotismus'') into biological context. Lichens have since been recognized as important actors in nutrient cycling and producers which many higher trophic feeders feed on, such as reindeer, gastropods, nematodes, mites, and springtails. Lichens have properties different from those of their component organisms. They come in man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serpentinite
Serpentinite is a metamorphic rock composed predominantly of serpentine group minerals formed by serpentinization of mafic or ultramafic rocks. The ancient origin of the name is uncertain; it may be from the similarity of its texture or color to snake skin. Greek pharmacologist Pedanius Dioscorides, Dioscorides (AD 50) recommended this rock to prevent snakebite. Serpentinite has been called ''serpentine'' or ''serpentine rock'', particularly in older geological texts and in wider cultural settings.California Government Code § 425.2; ''see'' Most of the chemical reactions necessary to synthesize acetyl-CoA, essential to basic biochemical pathways of life, take place during serpentinization. Serpentinite thermal vents are therefore considered a candidate for the origin of life on Earth. Formation and mineralogy Serpentinite is formed by near to complete serpentinization of mafic or ultramafic rocks. Serpentinite is formed from mafic rock that is mineral hydration, hydrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalate Minerals
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as dimethyl oxalate (). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate. Relationship to oxalic acid The dissociation of protons from oxalic acid proceeds in a determined order; as for other polyprotic acids, loss of a single proton results in the monovalent hydrogenoxalate anion . A salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant ( ''K''a) for loss of the first proton is ( p''K''a = 1.27). The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of (p''K''a = 4.28). These values imply, in solutions with neutral pH, no oxalic acid and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Minerals
Magnesium is a chemical element; it has symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table), it occurs naturally only in combination with other elements and almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrate Minerals
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are not inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic Minerals
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism (geometry), prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. The length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal class ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |