|
Galvanic Pain
{{Disambiguation ...
Galvanic (after Luigi Galvani) may refer to: * Galvanic anode * Galvanic bath * Galvanic cell * Galvanic corrosion * Galvanic current * Galvanic isolation * Galvanic potential * Galvanic series * Galvanic skin response * Galvanic vestibular stimulation * Galvanism * Galvanization * Operation Galvanic, World War II attack which included the Battle of Tarawa See also * List of forms of electricity named after scientists This is a list of forms of electricity named after scientists. The terms in this list are mostly archaic usages but are found in many 19th and early 20th-century publications. Adjectives ; faradic : Of electricity that is alternating, especial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luigi Galvani
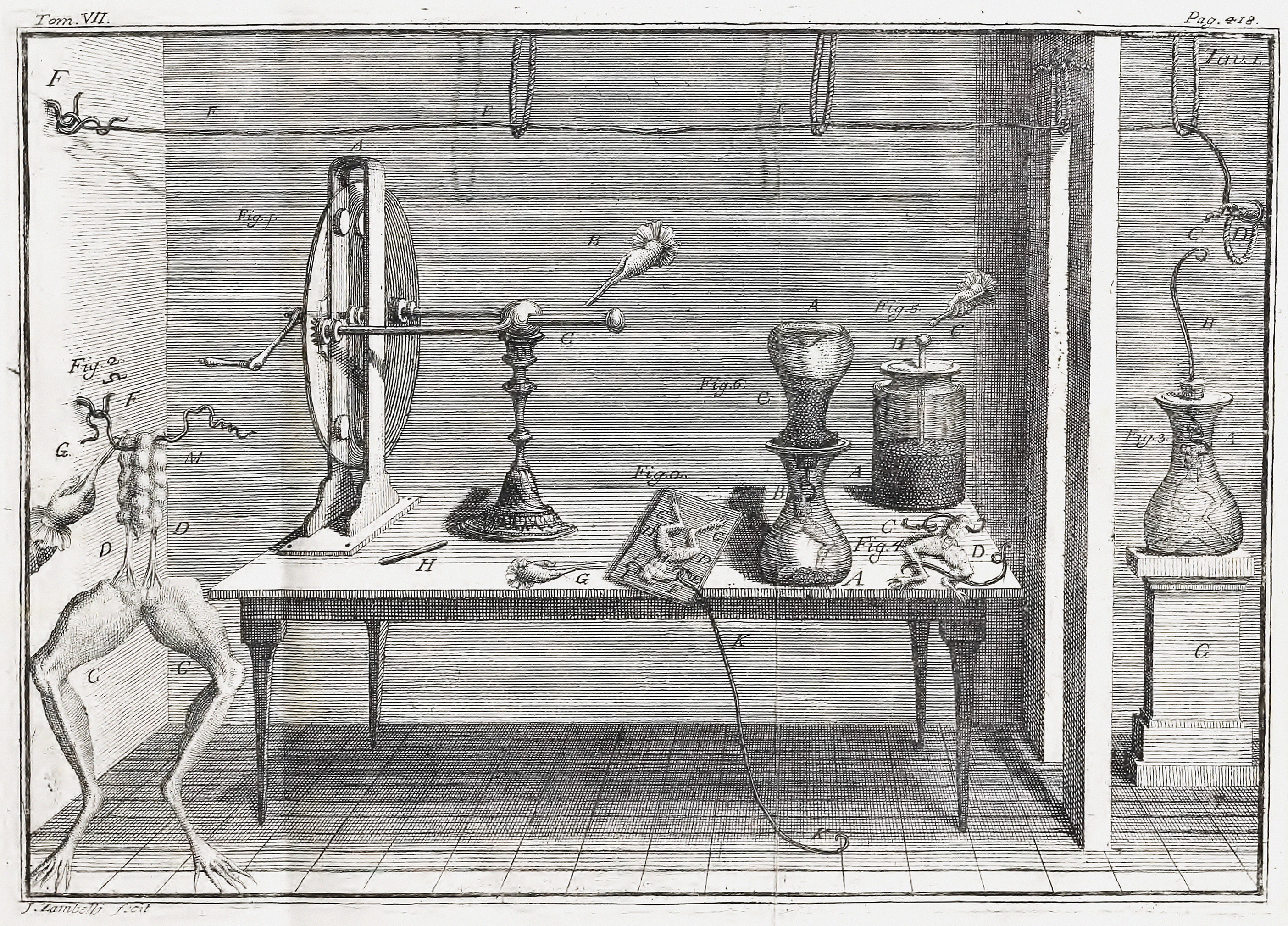
Luigi Galvani ( , , ; ; 9 September 1737 – 4 December 1798) was an Italian physician, physicist, biologist and philosopher who studied animal electricity. In 1780, using a frog, he discovered that the muscles of dead frogs' legs twitched when struck by an electrical spark. This was an early study of bioelectricity, following experiments by John Walsh (scientist), John Walsh and Hugh Williamson. Early life Luigi Galvani was born to Domenico Galvani and Barbara Caterina Foschi, in Bologna, then part of the Papal States. The house in which he was born may still be seen on Via Marconi, 25, in the center of Bologna. Domenico was a goldsmith. His family had produced several illustrious men. Galvani then began taking an interest in the field of "medical electricity". This field emerged in the middle of the 18th century, following electrical researches and the discovery of the effects of electricity on the human body by scientists including Bertrand Bajon and Ramón María Termey ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Anode
A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged metal structures from corrosion. They are made from a metal alloy with a more "active" voltage (more negative reduction potential / more positive oxidation potential) than the metal of the structure. The difference in potential between the two metals means that the galvanic anode corrodes, in effect being "sacrificed" in order to protect the structure. Theory In brief, corrosion is a chemical reaction occurring by an electrochemical mechanism (a redox reaction).Shrier 10:4 During corrosion of iron or steel there are two reactions, oxidation (equation ), where electrons leave the metal (and the metal dissolves, i.e. actual loss of metal results) and reduction, where the electrons are used to convert oxygen and water to hydroxide ions (equation ): In most environments, the hydroxide ions and ferrous ions combine to form ferrous hydroxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Bath
Galvanic bath is an alternative medical treatment (a type of electrotherapy) based on the simultaneous use of water and electric current. The patient lies in a 34 degree Celsius bath, while gentle electric current is passed through his body. Galvanic baths are mostly used in the treatment of degenerative diseases such as inflammatory arthritis and problems with the joints. The treatment lasts about 15 minutes. Types In addition to full galvanic baths, when the patient's body is fully immersed in water, there are also ''four-chambered galvanic baths'' (also called ''four cell galvanic baths''), which also combine electrical energy and hydrotherapy, but are used only on the limbs. Patient's forearms and lower legs are put in water trays, and electric current is passed through warm water. This procedure is said to improve the circulation, reduce pain and was considered especially beneficial for rheumatoid arthritis though no studies have been done to confirm these claims. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane. Volta was the inventor of the voltaic pile, the first electrical battery. Common usage of the word ''battery'' has evolved to include a single Galvanic cell, but the first batteries had many Galvanic cells. History In 1780, Luigi Galvani discovered that when two different metals (e.g., copper and zinc) are in contact and then both are touched at the same time to two different parts of a muscle of a frog leg, to close the circuit, the frog's leg contracts. He called this " animal electricity". The frog's leg, as well as being a detector ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Corrosion
Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, different metal, when both in the presence of an electrolyte. A similar galvanic reaction is exploited in single-use battery cells to generate a useful electrical voltage to power portable devices. This phenomenon is named after Italian physician Luigi Galvani (1737–1798). A similar type of corrosion caused by the presence of an external electric current is called '' electrolytic corrosion''. Overview Dissimilar metals and alloys have different electrode potentials, and when two or more come into contact in an electrolyte, one metal (that is more reactive) acts as anode and the other (that is less reactive) as cathode. The electropotential difference between the reactions at the two electrodes is the driving force for an accelerated attack on the anode metal, which disso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Current
Direct current (DC) is one-directional flow of electric charge. An electrochemical cell is a prime example of DC power. Direct current may flow through a conductor such as a wire, but can also flow through semiconductors, insulators, or even through a vacuum as in electron or ion beams. The electric current flows in a constant direction, distinguishing it from alternating current (AC). A term formerly used for this type of current was galvanic current. The abbreviations ''AC'' and ''DC'' are often used to mean simply ''alternating'' and ''direct'', as when they modify ''current'' or ''voltage''. Direct current may be converted from an alternating current supply by use of a rectifier, which contains electronic elements (usually) or electromechanical elements (historically) that allow current to flow only in one direction. Direct current may be converted into alternating current via an inverter. Direct current has many uses, from the charging of batteries to large power supp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Isolation
Galvanic isolation is a principle of isolating functional sections of electrical systems to prevent current flow; no direct conduction path is permitted. Energy or information can still be exchanged between the sections by other means, such as capacitive, inductive, radiative, optical, acoustic, or mechanical coupling. Galvanic isolation is used where two or more electric circuits must communicate, but their grounds may be at different potentials. It is an effective method of breaking ground loops by preventing unwanted current from flowing between two units sharing a ground conductor. Galvanic isolation is also used for safety, preventing accidental electric shocks. Methods Transformer Transformers are probably the most common means of galvanic isolation. They are almost universally used in power supplies because they are a mature technology that can carry significant power. They are also used to isolate data signals in Ethernet over twisted pair. Transformers co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode Potential
An electrode is an electrical conductor used to make contact with a nonmetallic part of a Electronic circuit, circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes. Michael Faraday coined the term "" in 1833; the word recalls the Greek ἤλεκτρον (, "amber") and ὁδός (, "path, way"). The Electrophorus, electrophore, invented by Johan Wilcke in 1762, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any Electric battery, battery. The first electrochemical battery was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Series
The galvanic series (or electropotential series) determines the nobility of metals and semi-metals. When two metals are submerged in an electrolyte, while also electrically connected by some external conductor, the less noble (base) will experience galvanic corrosion. The rate of corrosion is determined by the electrolyte, the difference in nobility, and the relative areas of the anode and cathode exposed to the electrolyte. The difference can be measured as a difference in voltage potential: the less noble metal is the one with a lower (that is, more negative) electrode potential than the nobler one, and will function as the anode (electron or anion attractor) within the electrolyte device functioning as described above (a galvanic cell). Galvanic reaction is the principle upon which batteries are based. See the '' table'' of standard electrode potentials for more details. Galvanic series (most noble at top) The following is the galvanic series for stagnant (that is, low oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Skin Response
Electrodermal activity (EDA) is the property of the human body that causes continuous variation in the electrical characteristics of the skin. Historically, EDA has also been known as skin conductance, galvanic skin response (GSR), electrodermal response (EDR), psychogalvanic reflex (PGR), skin conductance response (SCR), sympathetic skin response (SSR) and skin conductance level (SCL). The long history of research into the active and passive electrical properties of the skin by a variety of disciplines has resulted in an excess of names, now standardized to electrodermal activity (EDA). The traditional theory of EDA holds that skin resistance varies with the state of sweat glands in the skin. Sweating is controlled by the sympathetic nervous system, and skin conductance is an indication of psychological or physiological arousal. If the sympathetic branch of the autonomic nervous system is highly aroused, then sweat glands activity also increases, which in turn increases skin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanic Vestibular Stimulation
Galvanic vestibular stimulation is the process of sending specific electric messages to a nerve in the ear that maintains balance. There are two main groups of receptors in the vestibular system: the three semi-circular canals, and the two otolith organs (the Utricle (ear), utricle and the saccule). This technology has been investigated for both military and commercial purposes. The technology is being applied in Atsugi, Kanagawa, Atsugi, Japan, the Mayo Clinic in the US, and a number of other research institutions around the world. It is being investigated for a variety of applications, including biomedical, pilot training, and entertainment. Not much is known about galvanic vestibular stimulation, but more scientists are continuing to research the topic. A patient undergoing GVS noted: I felt a mysterious, irresistible urge to start walking to the right whenever the researcher turned the switch to the right. I was convinced—mistakenly—that this was the only way to mainta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galvanism
Galvanism is a term invented by the late 18th-century physicist and chemist Alessandro Volta to refer to the generation of electric current by chemical action. The term also came to refer to the discoveries of its namesake, Luigi Galvani, specifically the generation of electric current within biological organisms and the contraction/convulsion of biological muscle tissue upon contact with electric current. While Volta theorized and later demonstrated the phenomenon of his "Galvanism" to be replicable with otherwise inert materials, Galvani thought his discovery to be a confirmation of the existence of "animal electricity," a vital force which gave life to organic matter. History Johann Georg Sulzer Galvanic phenomena were described in the literature before it was understood that they were of an electrical nature. In 1752, when the Swiss mathematician and physicist Johann Georg Sulzer placed his tongue between a piece of lead and a piece of silver, joined at their edges, he pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |