|
Fluor-liddicoatite
Fluor-liddicoatite is a rare member of the tourmaline group of minerals, elbaite subgroup, and the theoretical calcium endmember of the elbaite-fluor-liddicoatite series; the pure end-member has not yet been found in nature. Fluor-liddicoatite is indistinguishable from elbaite by X-ray diffraction techniques. It forms a series with elbaite and probably also with olenite. Liddiocoatite is currently a non-approved mineral name, but Aurisicchio et al. (1999) and Breaks et al. (2008) found OH-dominant species. Formulae are * Fluor-liddicoatite Ca(Li2Al)Al6(BO3)3Si6O18(OH)3F * Elbaite Na(Al1.5Li1.5)Al6(BO3)3Si6O18(OH)4 * Olenite NaAl9B3Si6O27O3(OH) Fluor-liddicoatite was named in 1977 after Richard T. Liddicoat (1918–2002) gemmologist and president of the Gemological Institute of America, who is well known for introducing the GIA diamond grading system in 1953. Unit cell Fluor-liddicoatite belongs to the trigonal crystal system, class 3 m, space group R 3m. It has a rhombohedr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elbaite
Elbaite, a sodium, lithium, aluminium boro- silicate, with the chemical composition Na(Li1.5Al1.5)Al6Si6O18(BO3)3(OH)4, is a mineral species belonging to the six-member ring cyclosilicate tourmaline group. Elbaite forms three series, with dravite, with fluor-liddicoatite, and with schorl. Due to these series, specimens with the ideal endmember formula are not found occurring naturally. As a gemstone, elbaite is a desirable member of the tourmaline group because of the variety and depth of its colours and quality of the crystals. Originally discovered on the island of Elba, Italy in 1913, it has since been found in many parts of the world. In 1994, a major locality was discovered in Canada, at O'Grady Lakes in the Yukon. Elbaite forms in igneous and metamorphic rocks and veins in association with lepidolite, microcline, and spodumene in granite pegmatites; with andalusite and biotite in schist; and with molybdenite and cassiterite in massive hydrothermal replacement dep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tourmaline
Tourmaline ( ) is a crystalline silicate mineral group in which boron is compounded with elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. Tourmaline is a gemstone and can be found in a wide variety of colors. The term is derived from the Sinhalese "tōramalli", which refers to the carnelian gemstones. History Brightly colored Ceylonese gem tourmalines were brought to Europe in great quantities by the Dutch East India Company to satisfy a demand for curiosities and gems. Tourmaline was sometimes called the "Ceylonese Magnet" because it could attract and then repel hot ashes due to its pyroelectric properties. Tourmalines were used by chemists in the 19th century to polarize light by shining rays onto a cut and polished surface of the gem. Species and varieties Commonly encountered species and varieties: Schorl species: : Brownish black to black—''schorl'', Dravite species: from the Drave district of Carinthia : Dark yellow to brownis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
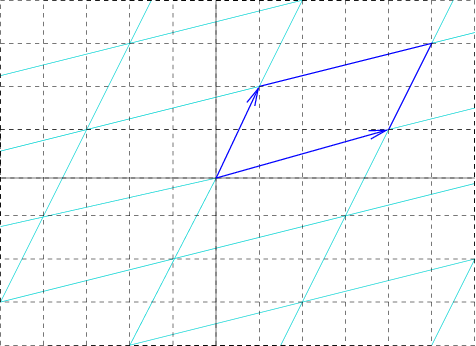
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type Locality (geology)
Type locality, also called type area, is the locality where a particular rock type, stratigraphic unit or mineral species is first identified. If the stratigraphic unit in a locality is layered, it is called a stratotype, whereas the standard of reference for unlayered rocks is the type locality. The term is similar to the term type site in archaeology or the term type specimen in biology. Examples of geological type localities Rocks and minerals * Aragonite: Molina de Aragón, Guadalajara, Spain * Autunite: Autun, France * Benmoreite: Ben More (Mull), Scotland * Blairmorite: Blairmore, Alberta, Canada * Boninite: Bonin Islands, Japan * Comendite: Comende, San Pietro Island, Sardinia * Cummingtonite: Cummington, Massachusetts * Dunite: Dun Mountain, New Zealand. * Essexite: Essex County, Massachusetts, US * Fayalite: Horta, Fayal Island, Azores, Portugal * Harzburgite: Bad Harzburg, Germany * Icelandite: Thingmuli (Þingmúli), Iceland * Ijolite: Iivaa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyramid (geometry)
In geometry, a pyramid () is a polyhedron formed by connecting a polygonal base and a point, called the apex (geometry), apex. Each base edge and apex form a triangle, called a ''lateral face''. It is a cone, conic solid with polygonal base. A pyramid with an base has Vertex (geometry), vertices, Face (geometry), faces, and Edge (geometry), edges. All pyramids are Self-dual polyhedron, self-dual. A right pyramid has its apex directly above the centroid of its base. Nonright pyramids are called oblique pyramids. A regular pyramid has a regular polygon base and is usually implied to be a ''right pyramid''. When unspecified, a pyramid is usually assumed to be a ''regular'' square pyramid, like the physical pyramid structures. A triangle-based pyramid is more often called a tetrahedron. Among oblique pyramids, like acute and obtuse triangles, a pyramid can be called ''acute'' if its apex is above the interior of the base and ''obtuse'' if its apex is above the exterior of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of partic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal Crystal System
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhombohedral. Each lattice system consists of one Bravais l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prism (geometry)
In geometry, a prism is a polyhedron comprising an polygon base, a second base which is a translated copy (rigidly moved without rotation) of the first, and other faces, necessarily all parallelograms, joining corresponding sides of the two bases. All cross-sections parallel to the bases are translations of the bases. Prisms are named after their bases, e.g. a prism with a pentagonal base is called a pentagonal prism. Prisms are a subclass of prismatoids. Like many basic geometric terms, the word ''prism'' () was first used in Euclid's Elements. Euclid defined the term in Book XI as “a solid figure contained by two opposite, equal and parallel planes, while the rest are parallelograms”. However, this definition has been criticized for not being specific enough in relation to the nature of the bases, which caused confusion among later geometry writers. Oblique prism An oblique prism is a prism in which the joining edges and faces are ''not perpendicular'' to the bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is a meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge length of the cube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |