|
FLiBe
FLiBe is a molten salt made from a mixture of lithium fluoride (LiF) and beryllium fluoride (). It is both a nuclear reactor coolant and solvent for fertile or fissile material. It served both purposes in the Molten-Salt Reactor Experiment (MSRE) at the Oak Ridge National Laboratory. The 2:1 molar mixture forms a stoichiometric compound, ( lithium tetrafluoroberyllate), which has a melting point of , a boiling point of , and a density of . Its volumetric heat capacity, 4540 kJ/(m3·K), is similar to that of water, more than four times that of sodium, and more than 200 times that of helium at typical reactor conditions. Its specific heat capacity is 2414.17 J/(kg·K), or about 60% that of water. Its appearance is white to transparent, with crystalline grains in a solid state, morphing into a completely clear liquid upon melting. However, soluble fluorides such as and , can dramatically change the salt's color in both solid and liquid state. This made spectrophotometry a vi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molten-Salt Reactor Experiment
The Molten-Salt Reactor Experiment (MSRE) was an experimental molten-salt reactor research reactor at the Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee. This technology was researched through the 1960s, the reactor was constructed by 1964, it went critical in 1965, and was operated until 1969. The costs of a cleanup project were estimated at $130 million. Initially designed for 15 MWth, the MSRE was operated at 7.4 MWth because of imprecise nuclear cross section data. It was a test reactor simulating the neutronic "kernel" of a type of inherently safer epithermal thorium breeder reactor called the liquid fluoride thorium reactor. It primarily used two fuels: first uranium-235 and later uranium-233. The latter 233UF4 was the result of breeding from thorium in other reactors. Since this was an engineering test, the large, expensive breeding blanket of thorium salt was omitted in favor of neutron measurements. In the MSRE, the heat from the reactor core was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Fluoride
Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Partly because Li and F are both light elements, and partly because is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO. Manufacturing LiF is prepared from lithium hydroxide or lithium carbonate with hydrogen fluoride. Applications Precursor to lithium hexafluorophosphate for batteries Lithium fluoride is reacted with hydrogen fluoride (HF) and phosphorus pentachloride to make lithium hexafluorophosphate , an ingredient in lithium ion battery electrolyte. The lithium fluoride alone does not absorb hydrogen fluoride to form a bifluoride salt. In molten salts Fluorine is produced by the elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beryllium Fluoride
Beryllium fluoride is the inorganic compound with the formula Be F2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water. Properties Beryllium fluoride has distinctive optical properties. In the form of fluoroberyllate glass, it has the lowest refractive index In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refrac ... for a solid at room temperature of 1.275. Its dispersive power is the lowest for a solid at 0.0093, and the nonlinear coefficient is also the lowest at 2 × 10−14. Structure and bonding The structure of solid BeF2 resembles that of cristobalite. Be2+ centers are four coordinate and tetrahedral and the fluoride centers are two-coordinate. The Be-F bond lengths a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor Coolant
A nuclear reactor coolant is a coolant in a nuclear reactor used to remove heat from the nuclear reactor core and transfer it to electrical generators and the environment. Frequently, a chain of two coolant loops are used because the primary coolant loop takes on short-term radioactivity from the reactor. Water Almost all currently operating nuclear power plants are light water reactors using ordinary water under high pressure as coolant and neutron moderator. About 1/3 are boiling water reactors where the primary coolant undergoes phase transition to steam inside the reactor. About 2/3 are pressurized water reactors at even higher pressure. Current reactors stay under the critical point at around 374 °C and 218 bar where the distinction between liquid and gas disappears, which limits thermal efficiency, but the proposed supercritical water reactor would operate above this point. Heavy water reactors use deuterium oxide which has identical properties to ordinary water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molten Salt
Molten salt is salt which is solid at standard temperature and pressure but liquified due to elevated temperature. A salt that is liquid even at standard temperature and pressure is usually called a room-temperature ionic liquid, and molten salts are technically a class of ionic liquids. Examples As a reference, molten sodium chloride, table salt has a melting point (m.p.) of . A variety of eutectic mixtures have been developed with lower melting points: Chlorides *Lithium chloride and potassium chloride, m.p. . Nitrates Alkali metal nitrates are relatively low melting and thermally stable. The least stable, (m.p. ) decomposes only at . At the other extreme, cesium nitrate melts at and decomposes at 584 °C. *60:40 mixture of sodium nitrate and potassium nitrate is a liquid between . It has a heat of fusion of 161 J/g, and a heat capacity of 1.53 J/(g·K). *1:1 mixture :, m.p. . *40:7:53 ::, m. p. , stable to . Uses Molten salts have a variety of uses. Production of magnes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroberyllate
Tetrafluoroberyllate or orthofluoroberyllate is an anion with the chemical formula . It contains beryllium and fluorine. This fluoroanion has a tetrahedral shape, with the four fluorine atoms surrounding a central beryllium atom. It has the same size, charge, and outer electron structure as sulfate . Therefore, many compounds that contain sulfate have equivalents with tetrafluoroberyllate. Examples of these are the langbeinites, and Tutton's salts. Properties The Be–F bond length is between 145 and 153 pm. The beryllium is sp3 hybridized, leading to a longer bond than in , where beryllium is sp hybridized. In trifluoroberyllates, there are actually tetrahedra arranged in a triangle, so that three fluorine atoms are shared on two tetrahedra each, resulting in a formula of . In the tetrafluoroberyllates, the tetrahedra can rotate to various degrees. At room temperature, they are hindered from moving. But as temperature increases, they can rotate around the threefold ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
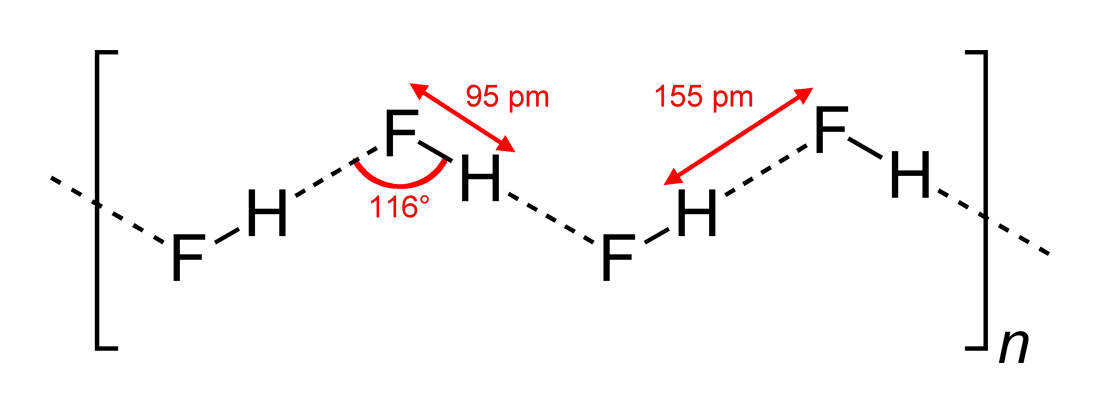
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactivity (chemistry), reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. It exhibits a metallic luster (mineralogy), luster. It corrosion, corrodes quickly in air to a dull silvery gray, then black tarnish. It does not occur freely in nature, but occurs mainly as pegmatite, pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolysis, electrolytically from a mixture of lithium chloride and potassium chloride. The Atomic nucleus, nucleus of the lithiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beryllium Hydroxide
Beryllium hydroxide, Be(OH)2, is an amphoteric hydroxide, dissolving in both acids and alkalis. Industrially, it is produced as a by-product in the extraction of beryllium metal from the ores beryl and bertrandite. The natural pure beryllium hydroxide is rare (in form of the mineral behoite, orthorhombic) or very rare (clinobehoite, monoclinic).Mindat, http://www.mindat.org/min-1066.html When alkali is added to beryllium salt solutions the α-form (a gel) is formed. If this left to stand or boiled, the rhombic β-form precipitates.Mary Eagleson, 1994, Concise encyclopedia chemistry, Walter de Gruyter, This has the same structure as zinc hydroxide, Zn(OH)2, with tetrahedral beryllium centers. Reactions Beryllium hydroxide is difficult to dissolve in water. With alkalis it dissolves to form the tetrahydroxoberyllate (also known as tetrahydroxidoberyllate) anion, e(OH)4sup>2−.Egon Wiberg, Arnold Frederick Holleman (2001) ''Inorganic Chemistry'', Elsevier With sodium hydroxide so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |