|
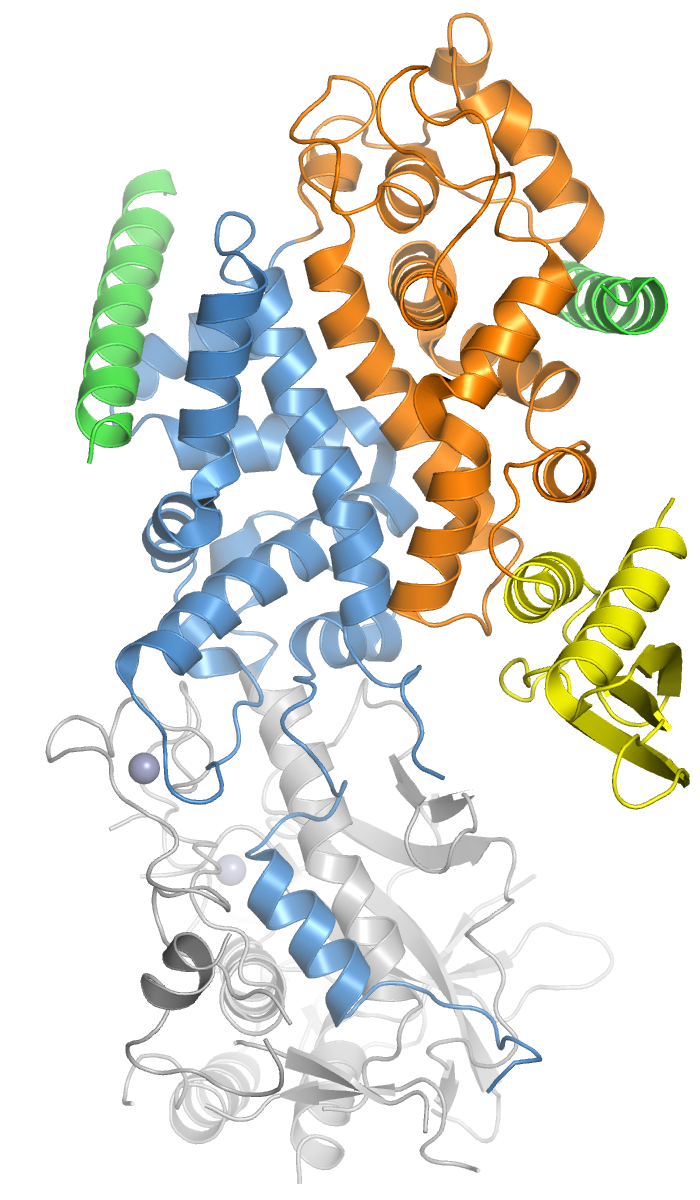
DROSHA
Drosha is a Class 2 ribonuclease III enzyme that in humans is encoded by the ''DROSHA'' (formerly ''RNASEN'') gene. It is the primary nuclease that executes the initiation step of miRNA processing in the nucleus. It works closely with DGCR8 and in correlation with Dicer. It has been found significant in clinical knowledge for cancer prognosisSlack FJ, Weidhaas JB (December 2008). "MicroRNA in cancer prognosis". ''The New England Journal of Medicine.'' 359 (25): 2720-2. and HIV-1 replication.Swaminathan, G., Navas-Martín, S., & Martín-García, J. (2014). MicroRNAs and HIV-1 infection: antiviral activities and beyond. ''Journal of molecular biology'', ''426''(6), 1178-1197. History Human Drosha was cloned in 2000 when it was identified as a nuclear dsRNA ribonuclease involved in the processing of ribosomal RNA precursors. The other two human enzymes that participate in the processing and activity of miRNA are the Dicer and Argonaute proteins. Recently, proteins like Drosha ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonuclease III
Ribonuclease III (RNase III or RNase C)(BREND3.1.26.3 is a type of ribonuclease that recognizes dsRNA and cleaves it at specific targeted locations to transform them into mature RNAs. These enzymes are a group of endoribonucleases that are characterized by their ribonuclease domain, which is labelled the RNase III domain. They are ubiquitous compounds in the cell and play a major role in pathways such as RNA precursor synthesis, RNA Silencing, and the ''pnp'' autoregulatory mechanism. Types of RNase III The RNase III superfamily is divided into four known classes: 1, 2, 3, and 4. Each class is defined by its domain structure.Liang Y-H, Lavoie M, Comeau M-A, Elela SA, Ji X. Structure of a Eukaryotic RNase III Post-Cleavage Complex Reveals a Double- Ruler Mechanism for Substrate Selection. Molecular cell. 2014;54(3):431-444. doi:10.1016/j.molcel.2014.03.006. Class 1 RNase III *Class 1 RNase III enzymes have a homodimeric structure whose function is to cleave dsRNA into multiple su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicer
Dicer, also known as endoribonuclease Dicer or helicase with RNase motif, is an enzyme that in humans is encoded by the gene. Being part of the RNase III family, Dicer cleaves double-stranded RNA (dsRNA) and pre-microRNA (pre-miRNA) into short double-stranded RNA fragments called small interfering RNA and microRNA, respectively. These fragments are approximately 20–25 base pairs long with a two-base overhang on the 3′-end. Dicer facilitates the activation of the RNA-induced silencing complex (RISC), which is essential for RNA interference. RISC has a catalytic component Argonaute, which is an endonuclease capable of degrading messenger RNA (mRNA). Discovery Dicer was given its name in 2001 by Stony Brook PhD student Emily Bernstein while conducting research in Gregory Hannon's lab at Cold Spring Harbor Laboratory. Bernstein sought to discover the enzyme responsible for generating small RNA fragments from double-stranded RNA. Dicer's ability to generate around ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microprocessor Complex
The microprocessor complex is a protein complex involved in the early stages of processing microRNA (miRNA) and RNA interference (RNAi) in animal cells. The complex is minimally composed of the ribonuclease enzyme Drosha and the dimeric RNA-binding protein DGCR8 (also known as Pasha in non-human animals), and cleaves primary miRNA substrates to pre-miRNA in the cell nucleus. Microprocessor is also the smaller of the two multi-protein complexes that contain human Drosha. Composition The microprocessor complex consists minimally of two proteins: Drosha, a ribonuclease III enzyme; and DGCR8, a double-stranded RNA binding protein. (DGCR8 is the name used in mammalian genetics, abbreviated from "DiGeorge syndrome critical region 8"; the homologous protein in model organisms such as flies and worms is called ''Pasha'', for ''Pa''rtner of Dro''sha''.) The stoichiometry of the minimal complex was at one point experimentally difficult to determine, but it has been demonstrated to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MicroRNA
MicroRNA (miRNA) are small, single-stranded, non-coding RNA molecules containing 21 to 23 nucleotides. Found in plants, animals and some viruses, miRNAs are involved in RNA silencing and post-transcriptional regulation of gene expression. miRNAs base-pair to complementary sequences in mRNA molecules, then gene silence said mRNA molecules by one or more of the following processes: (1) cleavage of mRNA strand into two pieces, (2) destabilization of mRNA by shortening its poly(A) tail, or (3) translation of mRNA into proteins. This last method of gene silencing is the least efficient of the three, and requires the aid of ribosomes. miRNAs resemble the small interfering RNAs (siRNAs) of the RNA interference (RNAi) pathway, except miRNAs derive from regions of RNA transcripts that fold back on themselves to form short hairpins, whereas siRNAs derive from longer regions of double-stranded RNA. The human genome may encode over 1900 miRNAs, although more recent analysis s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pasha (protein)
The microprocessor complex subunit DGCR8 ''( DiGeorge syndrome critical region 8)'' is a protein that in humans is encoded by the gene. In other animals, particularly the common model organisms ''Drosophila melanogaster'' and ''Caenorhabditis elegans'', the protein is known as ''Pasha'' (partner of Drosha). It is a required component of the RNA interference pathway. Function The subunit DGCR8 is localized to the cell nucleus and is required for microRNA (miRNA) processing. It binds to the other subunit Drosha, an RNase III enzyme, to form the microprocessor complex that cleaves a primary transcript known as pri-miRNA to a characteristic stem-loop structure known as a pre-miRNA, which is then further processed to miRNA fragments by the enzyme Dicer. DGCR8 contains an RNA-binding domain and is thought to bind pri-miRNA to stabilize it for processing by Drosha. DGCR8 is also required for some types of DNA repair. Removal of UV-induced DNA photoproducts, during transcript ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stem-loop
Stem-loop intramolecular base pairing is a pattern that can occur in single-stranded RNA. The structure is also known as a hairpin or hairpin loop. It occurs when two regions of the same strand, usually complementary in nucleotide sequence when read in opposite directions, base-pair to form a double helix that ends in an unpaired loop. The resulting structure is a key building block of many RNA secondary structures. As an important secondary structure of RNA, it can direct RNA folding, protect structural stability for messenger RNA (mRNA), provide recognition sites for RNA binding proteins, and serve as a substrate for enzymatic reactions. Formation and stability The formation of a stem-loop structure is dependent on the stability of the resulting helix and loop regions. The first prerequisite is the presence of a sequence that can fold back on itself to form a paired double helix. The stability of this helix is determined by its length, the number of mismatches or bulges i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multienzyme complexes, in which multiple catalytic domains are found in a single polypeptide chain. Protein complexes are a form of quaternary structure. Proteins in a protein complex are linked by non-covalent protein–protein interactions. These complexes are a cornerstone of many (if not most) biological processes. The cell is seen to be composed of modular supramolecular complexes, each of which performs an independent, discrete biological function. Through proximity, the speed and selectivity of binding interactions between enzymatic complex and substrates can be vastly improved, leading to higher cellular efficiency. Many of the techniques used to enter cells and isolate proteins are inherently disruptive to such large complexes, complicating the task of determining the components of a complex. Examples of protein complexes include th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DGCR8
The microprocessor complex subunit DGCR8 ''( DiGeorge syndrome critical region 8)'' is a protein that in humans is encoded by the gene. In other animals, particularly the common model organisms '' Drosophila melanogaster'' and ''Caenorhabditis elegans'', the protein is known as ''Pasha'' (partner of Drosha). It is a required component of the RNA interference pathway. Function The subunit DGCR8 is localized to the cell nucleus and is required for microRNA (miRNA) processing. It binds to the other subunit Drosha, an RNase III enzyme, to form the microprocessor complex that cleaves a primary transcript known as pri-miRNA to a characteristic stem-loop structure known as a pre-miRNA, which is then further processed to miRNA fragments by the enzyme Dicer. DGCR8 contains an RNA-binding domain and is thought to bind pri-miRNA to stabilize it for processing by Drosha. DGCR8 is also required for some types of DNA repair. Removal of UV-induced DNA photoproducts, during tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila
''Drosophila'' () is a genus of flies, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or (less frequently) pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species to linger around overripe or rotting fruit. They should not be confused with the Tephritidae, a related family, which are also called fruit flies (sometimes referred to as "true fruit flies"); tephritids feed primarily on unripe or ripe fruit, with many species being regarded as destructive agricultural pests, especially the Mediterranean fruit fly. One species of ''Drosophila'' in particular, '' D. melanogaster'', has been heavily used in research in genetics and is a common model organism in developmental biology. The terms "fruit fly" and "''Drosophila''" are often used synonymously with ''D. melanogaster'' in modern biological literature. The entire genus, however, contains more than 1,500 species and is very diverse in appea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Transcript
A primary transcript is the single-stranded ribonucleic acid (RNA) product synthesized by transcription of DNA, and processed to yield various mature RNA products such as mRNAs, tRNAs, and rRNAs. The primary transcripts designated to be mRNAs are modified in preparation for translation. For example, a precursor mRNA (pre-mRNA) is a type of primary transcript that becomes a messenger RNA (mRNA) after processing. Pre-mRNA is synthesized from a DNA template in the cell nucleus by transcription. Pre-mRNA comprises the bulk of heterogeneous nuclear RNA (hnRNA). Once pre-mRNA has been completely processed, it is termed "mature messenger RNA", or simply " messenger RNA". The term hnRNA is often used as a synonym for pre-mRNA, although, in the strict sense, hnRNA may include nuclear RNA transcripts that do not end up as cytoplasmic mRNA. There are several steps contributing to the production of primary transcripts. All these steps involve a series of interactions to initiate and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNA-binding Protein EWS
RNA-binding protein EWS is a protein that in humans is encoded by the ''EWSR1'' gene on human chromosome 22, specifically 22q12.2. It is one of 3 proteins in the FET protein family. The q22.2 region of chromosome 22 encodes the N-terminal transactivation domain of the EWS protein and that region may become joined to one of several other chromosomes which encode various transcription factors, see and the FET protein family. The expression of a chimeric protein with the EWS transactivation domain fused to the DNA binding region of a transcription factor generates a powerful oncogenic protein causing Ewing sarcoma and other members of the Ewing family of tumors. These translocations can occur due to chromoplexy, a burst of complex chromosomal rearrangements seen in cancer cells. The normal EWS gene encodes an RNA binding protein closely related to FUS (gene) and TAF15, all of which have been associated to amyotrophic lateral sclerosis. Interactions The EWS protein has been shown ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |