|
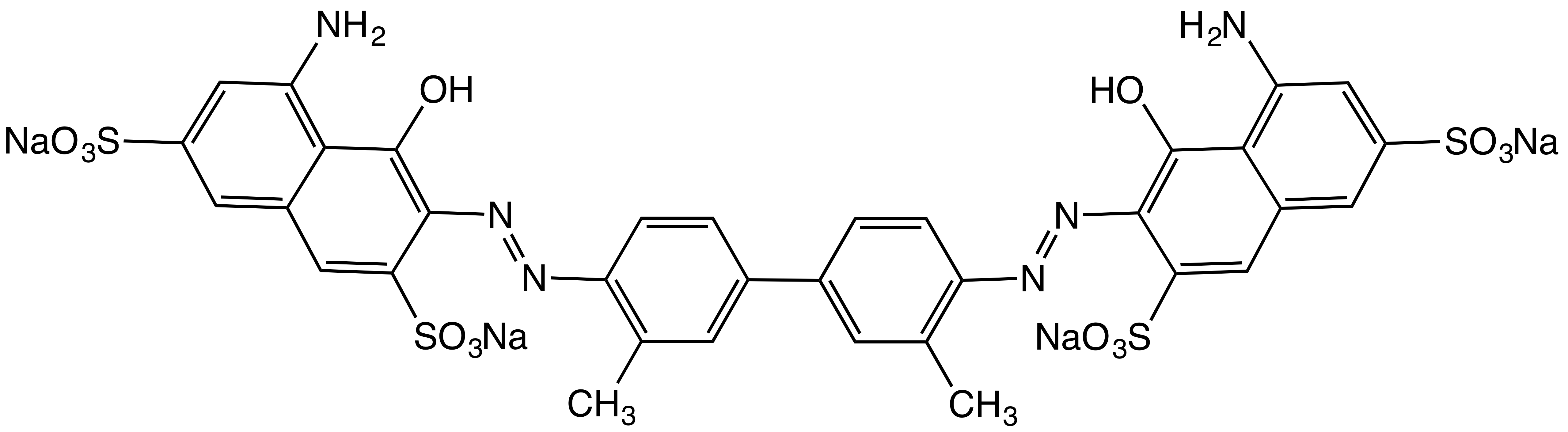
Disodium 4,4'-dinitrostilbene-2,2'-disulfonate
Disodium 4,4′-dinitrostilbene-2,2′-disulfonate is an organic compound with the formula (O2NC6H3(SO3Na)CH)2. This salt is a common precursor to a variety of textile dyes and optical brighteners. Preparation and reactions The synthesis of disodium 4,4′-dinitrostilbene-2,2′-disulfonate begins with sulfonation of 4-nitrotoluene. This reaction affords 4-nitrotoluene-2-sulfonic acid. Oxidation of this species with sodium hypochlorite yields the disodium salt of 4,4′-dinitrostilbene-2,2′-disulfonic acid. The product is useful as its reaction with aniline derivatives results in the formation of azo dyes. Commercially important dyes derived from this compound include Direct Red 76, Direct Brown 78, and Direct Orange 40. Reduction gives 4,4′-diamino-2,2′-stilbenedisulfonic acid, which is a common optical brightener. History Arthur Green and André Wahl first reported the formation of disodium 4,4'-dinitrostilbene-2,2'-disulfonate using sodium hypochlorite Sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Brightener
Optical brighteners, optical brightening agents (OBAs), fluorescent brightening agents (FBAs), or fluorescent whitening agents (FWAs), are chemical compounds that absorb light in the ultraviolet and violet region (usually 340-370 nm) of the electromagnetic spectrum, and re-emit light in the blue region (typically 420-470 nm) through the phenomenon of fluorescence. These additives are often used to enhance the appearance of color of fabric and paper, causing a "whitening" effect; they make intrinsically yellow/orange materials look less so, by compensating the deficit in blue and purple light reflected by the material, with the blue and purple optical emission of the fluorophore. Properties The most common classes of compounds with this property are the stilbenes, e.g., 4,4′-diamino-2,2′-stilbenedisulfonic acid. Older, non-commercial fluorescent compounds include umbelliferone, which absorbs in the UV portion of the spectrum and re-emit it in the blue portion of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonation
In organic chemistry, aromatic sulfonation is a reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid () group. Together with nitration and chlorination, aromatic sulfonation is a widely used electrophilic aromatic substitutions. Aryl sulfonic acids are used as detergents, dye, and drugs. : Stoichiometry and mechanism Typical conditions involve heating the aromatic compound with sulfuric acid: : Sulfur trioxide or its protonated derivative is the actual electrophile in this electrophilic aromatic substitution. To drive the equilibrium, dehydrating agents such as thionyl chloride can be added: : Historically, mercurous sulfate has been used to catalyze the reaction. Chlorosulfuric acid is also an effective agent: : In contrast to aromatic nitration and most other electrophilic aromatic substitutions this reaction is reversible. Sulfonation takes place in concentrated acidic conditions and desulfonation is the mode of action in a dilute hot aqueou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hypochlorite
Sodium hypochlorite is an alkaline inorganic chemical compound with the formula (also written as NaClO). It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations () and hypochlorite anions (, also written as and ). The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate , a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated. Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as chlorine bleach, which is a household chemical widely used (since the 18th century) as a disinfectant and bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is still the most important chlorine-based bleach. Its corrosive properties, common availability, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions ( anions), which results in a compound with no net electric charge (electrically neutral). The constituent ions are held together by electrostatic forces termed ionic bonds. The component ions in a salt can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic, such as ammonium () and carbonate () ions in ammonium carbonate. Salts containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases, such as sodium hydroxide and potassium oxide. Individual ions within a salt usually have multiple near neighbours, so they are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Salts usually form crystalline structures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for cellulose-based textil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ullmann's Encyclopedia Of Industrial Chemistry
''Ullmann's Encyclopedia of Industrial Chemistry'' is a major reference work related to Chemical industry, industrial chemistry by chemist Fritz Ullmann, first published in 1914, and exclusively in German as "Enzyklopädie der Technischen Chemie" until 1984. History Ullmann's Encyclopedia of Industrial Chemistry is a major reference work related to industrial chemistry by chemist Fritz Ullmann. Its first edition was published in German by Fritz Ullmann in 1914. The fourth edition, published 1972 to 1984, already contained 25 volumes. The fifth edition, published 1985 to 1996, was the first version available in English. In 1997, the first online version was published. The year 2014 marked its Centennial, centenary. Ullmann's Encyclopedia was in its seventh edition, in 40 volumes, including one index volume and more than 1,050 articles (200 more than the sixth edition), approximately 30,000 pages, 22,000 images, 8,000 tables, 19,000 references and 85,000 indices. Editions * 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wiley-VCH
Wiley-VCH is a German publisher owned by John Wiley & Sons. It was founded in 1921 as Verlag Chemie (meaning "Chemistry Press": VCH stands for ''Verlag Chemie'') by two German learned societies A learned society ( ; also scholarly, intellectual, or academic society) is an organization that exists to promote an academic discipline, profession, or a group of related disciplines such as the arts and sciences. Membership may be open to al .... Later, it was merged into the German Chemical Society (GDCh). In 1991, VCH acquired Akademie Verlag. It has been owned by John Wiley & Sons since 1996. The humanities section of Akademie Verlag and the Akademie brand were sold in 1997 to R. Oldenbourg Verlag, while VCH retained the natural sciences catalog. References External links * Wiley (publisher) Publishing companies of Germany Publishing companies established in 1921 Weinheim German companies established in 1921 {{publish-company-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chrysophenine
Chrysophenine is a yellow water-soluble disazo stilbene dye, also known as Direct Yellow 12 or Chrysophenine G. The chemical formula is . Synthesis Chrysophenine is obtained by reacting Brilliant Yellow with chloroethane, which converts the two hydroxyl groups of Brilliant Yellow into ether groups. In its pure state, the compound forms an orange powder, soluble in water. Uses The compound is widely used in textile dyeing, biological staining, and has potential applications in photodynamic therapy. Although it can contribute to water pollution, it can be effectively removed from wastewater through membrane filtration techniques. Chrysophenine is used as a direct dye, meaning it can directly stain fibers without the need for a mordant (a substance that helps fix the dye to the fiber). It gives cellulose materials (linen, cotton, paper, viscose, etc.) a bright golden-yellow color. The presence of sulfogroups in the molecules allows the dye to be used as an acidic dye for dyeing w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |