|
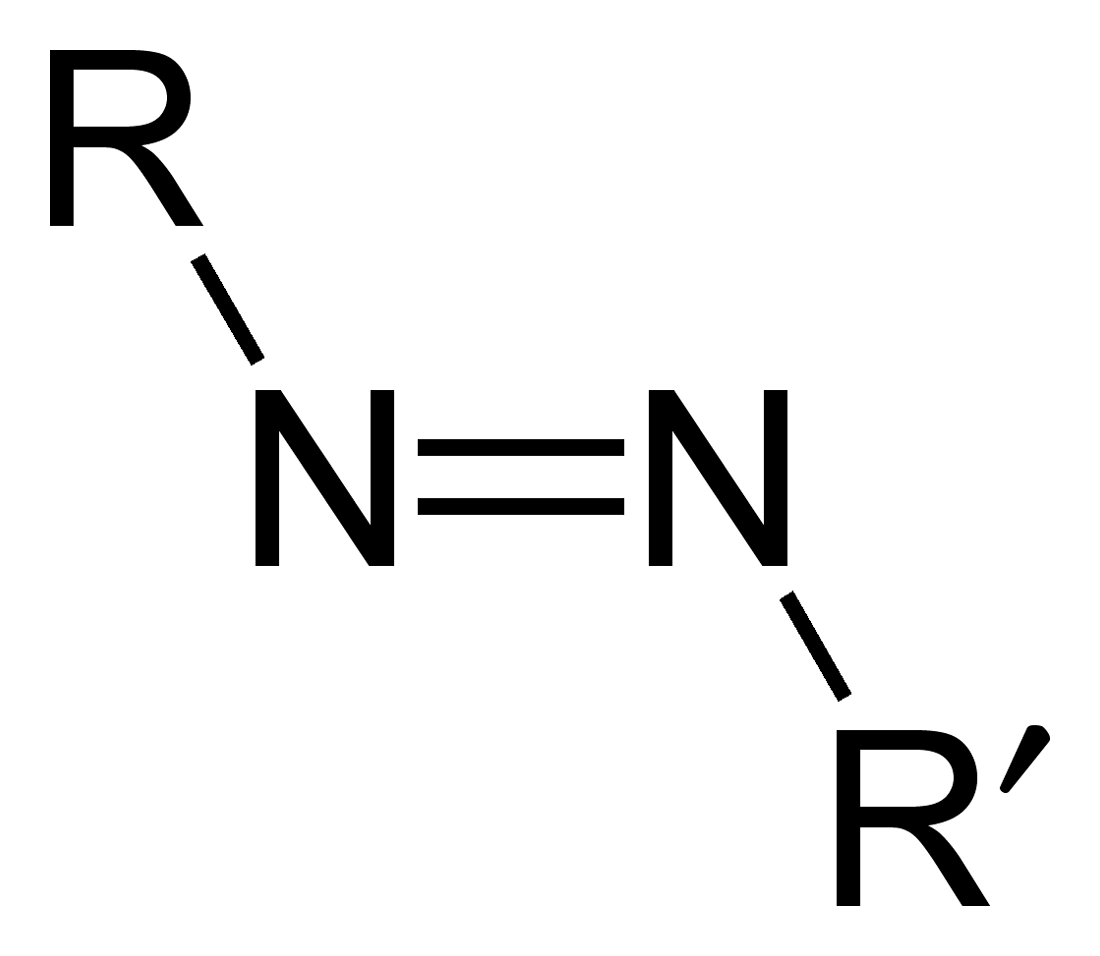
Diphosphene
Diphosphene is a compound having the formula . It exists as two geometric isomers, ''E'' and ''Z''. Diphosphene is also the parent member of the entire class of diphosphene compounds with the formula , where R is an organyl group. Visible radiation induces cis-trans isomerization, although further irradiation can excite the molecule to a triplet diradical state. In triplet trans-HPPH, the P-P bond length is predicted to be 2.291 Å. It is not only longer than the P-P double bond in ground state trans-bis(2,4,6-tri-tert-butylphenyl)diphosphene, but also longer than that of P-P single bond in . Calculation of the dihedral angle of trans-HPPH suggests that it is almost 90 degree, which means the formation of \pi and \pi^* P-P bonds is forbidden and σ bond is enhanced. References {{Inorganic-compound-stub Phosphorus hydrides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphenes
Diphosphene is a type of organophosphorus compound that has a phosphorus–phosphorus double bond, denoted by R-P=P-R'. These compounds are not common, but their properties have theoretical chemistry, theoretical importance. Normally, compounds with the empirical formula RP exist as rings. However, like other multiple bonds between heavy main-group elements, P=P double bonds can be stabilized by large steric effects, steric hindrance. In general, diphosphenes react like Alkene, alkenes. History In 1877, Köhler and Michaelis claimed what would have been the first isolated diphosphene (PhP=PPh), The structure of Köhler and Michaelis' product was later revised. and X-ray crystallographic analysis proved that this "diphosphene" only had P-P single bonds and was in fact primarily a four-membered ring of the form (PPh)4. The isolation of phosphorus ylide and phosphaalkenes suggested that compounds with P=P bonds could be made. Yoshifuji ''et al'''s isolated a sterically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing Polymeric foam, polymer foams, but applications also include its uses as a precursor (chemistry), precursor to pharmaceuticals and agrochemicals, as well as a long-term storable propellant for in-outer space, space spacecraft propulsion. Additionally, hydrazine is used in various rocket propellant, rocket fuels and to prepare the gas precursors used in airbags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. , approximately 120,000 tons of hydrazine hydrate (corresponding to a 64% solution of hydrazine in water by weight) we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazane
Triazane is an inorganic compound with the chemical formula or . Triazane is the third simplest acyclic azane after ammonia and hydrazine. It can be synthesized from hydrazine but is unstable and cannot be isolated in the free base form, only as salt forms such as triazanium sulfate. Attempts to convert triazanium salts to the free base release only diazene and ammonia. Triazane was first synthesized as a ligand In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ... of the silver complex ion: tris(μ2-triazane-κ2''N''1,''N''3)disilver(2+). Triazane has also been synthesized in electron-irradiated ammonia ices and detected as a stable gas-phase product after sublimation.Förstel, Maksyutenko, Jones, Sun, Chen, Chang, & Kaiser. "Detection of the Elusive Triazane Molecule () in the Ga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazene
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene. Synthesis A traditional route to diimide involves oxidation of hydrazine with hydrogen peroxide or air. : Alternatively the hydrolysis of diethyl azodicarboxylate or azodicarbonamide affords diimide: : Nowadays, diimide is generated by thermal decomposition of 2,4,6‐triisopropylbenzenesulfonylhydrazide. Because of its instability, diimide is generated and used ''in-situ''. A mixture of both the ''cis'' (''Z-'') and ''trans'' (''E-'') isomers is produced. Both isomers are unstable, and they undergo a slow interconversion. The ''trans'' isomer is more stable, but the ''cis'' isomer is the one that reacts with unsaturated substrates, therefore the equilibrium between them shifts towards the ''cis'' isomer du ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azene
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene.", where Ph stands for phenyl group. The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds urinary tract infections">Phenazopyridine, an aryl azo compound, is used to treat urinary tract infections">150px Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazene
Triazene is an unsaturated inorganic compound having the chemical formula N3 H3. It has one double bond and is the second-simplest member of the azene class of hydronitrogen compounds, after diimide. Triazenes are a class of organic compounds containing the functional group −N(H)−N=N−. Triazene, possibly along with its isomer triimide (HNNHNH), has been synthesized in electron-irradiated ices of ammonia and ammonia/dinitrogen and detected in the gas phase after sublimation. References External links *IUPAC Gold Book The International Union of Pure and Applied Chemistry (IUPAC) publishes many books which contain its complete list of definitions. The definitions are divided initially into seven IUPAC Colour Books: Gold, Green, Blue, Purple, Orange, White, and R ...br>definition {{Hydrides by group Nitrogen hydrides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazene
Tetrazene is a chemical compound with the molecular formula H2NN=NNH2. It is a colorless explosive material. An analogue is the organosilicon derivative (tms)2NN=NN(tms)2 where tms is trimethylsilyl. Isomeric with tetrazine is ammonium azide. Tetrazene explosive, commonly known simply as tetrazene, is used for sensitization of priming compositions. Properties Tetrazene has eleven isomers. The most stable of these is the straight-chain 2-tetrazene (H2N-N=N-NH2), having a standard heat of formation at 301.3 kJ/mol. The eleven isomers can be arranged into three groups: straight-chain tetrazenes, four-membered cyclotetrazane, and three-membered cyclotriazanes. Each straight-chain tetrazene isomer possesses one N=N double bond and two N-N single bonds. Tautomerizations do occur between the isomers. The ionic compound ammonium azide is also a constitutional isomer of tetrazene. Organometallic derivatives A variety of coordination complex A coordination complex is a chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an List of extremely hazardous substances, extremely hazardous substance. Ammonia is toxic, cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geometric Isomer
Geometry (; ) is a branch of mathematics concerned with properties of space such as the distance, shape, size, and relative position of figures. Geometry is, along with arithmetic, one of the oldest branches of mathematics. A mathematician who works in the field of geometry is called a '' geometer''. Until the 19th century, geometry was almost exclusively devoted to Euclidean geometry, which includes the notions of point, line, plane, distance, angle, surface, and curve, as fundamental concepts. Originally developed to model the physical world, geometry has applications in almost all sciences, and also in art, architecture, and other activities that are related to graphics. Geometry also has applications in areas of mathematics that are apparently unrelated. For example, methods of algebraic geometry are fundamental in Wiles's proof of Fermat's Last Theorem, a problem that was stated in terms of elementary arithmetic, and remained unsolved for several centuries. During t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organyl
In organic and organometallic chemistry, an organyl group (commonly denoted by the letter " R") is an organic substituent with one (sometimes more) free valence electron(s) at a carbon atom.. The term is often used in chemical patent literature to protect claims over a broad scope. Examples * Acetonyl group * Acyl group (e.g. acetyl group, benzoyl group) * Alkyl group (e.g., methyl group, ethyl group) * Alkenyl group (e.g., vinyl group, allyl group) * Alkynyl group ( propargyl group) * Benzyloxycarbonyl group (Cbz) * '' tert'' -butoxycarbonyl group (Boc) * Carboxyl group In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g. ... References Functional groups {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diradical
In chemistry, a diradical is a chemical species, molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate energy level, degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactivity (chemistry), reactive and non-Kekulé molecules that are rarely isolated. Diradicals are even-electron molecules but have one fewer chemical bond, bond than the number permitted by the octet rule. Examples of diradical species can also be found in coordination chemistry, for example among metal dithiolene complex, bis(1,2-dithiolene) metal complexes. Spin states Diradicals are usually triplet state, triplets. The phrases ''singlet'' and ''triplet'' are derived from the multiplicity of states of diradicals in electron spin resonance: a singlet diradical has one state (S=0, Ms=2*0+1=1, ms=0) and exhibits no signal in electron paramagnetic resonance, EPR and a triplet diradical has 3 states (S=1, Ms=2*1+1= ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |