|
Diazirine
Diazirines are a class of organic molecules consisting of a carbon bound to two nitrogen atoms, which are double-bonded to each other, forming a cyclopropene-like ring, 3''H''-diazirene. They are isomeric with diazocarbon groups, and like them can serve as precursors for carbenes by loss of a molecule of dinitrogen. For example, irradiation of diazirines with ultraviolet light leads to carbene insertion into various C-H, N-H, and O-H bonds. Hence, diazirines have grown in popularity as small photo-reactive crosslinking reagents. They are often used in photoaffinity labeling studies to observe a variety of interactions, including ligand-receptor, ligand-enzyme, protein-protein, and protein-nucleic acid interactions. Synthesis A number of methods exist in the literature for the preparation of diazirines, which begin from a variety of reagents. Synthesis from ketones Generally, synthetic schemes that begin with ketones involve conversion of the ketone with the desired substi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graham Reaction
In organic chemistry, the Graham reaction is an oxidation reaction that converts an amidine into a diazirine using a hypohalite reagent. The halide of the hypohalite oxidant, or another similar anionic additive to the reaction, is retained as a substituent on the diazirine product. The reaction was first reported in 1965. Various reaction mechanisms have been proposed. : Amidine substrates for the reaction can easily be formed from the corresponding nitriles via the Pinner reaction. The halide substituent in the diazirine product can be displaced by a various nucleophile In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...s. : References {{Organic-chem-stub Ring forming reactions Organic oxidation reactions Amidines Diazirines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diaziridine
Diaziridines are heterocyclic compounds containing two nitrogen atoms in a three-membered ring. They can be considered as strained hydrazines. Unlike most amine types of structures, the nitrogen atoms of diaziridines are configurationally stable because the ring strain prevents Walden inversion. As a result, there can be various stereoisomeric forms of this structure. They are usually synthesized by treating a carbonyl compound with an aminating reagent like hydroxylamine-''O''-sulfonic acid and either ammonia or a primary aliphatic amine under slightly basic conditions.Synthesis of monocyclic diaziridines and their fused derivatives; N. N. Makhova, V. Y. Petukhova, V. V. Kuznetsov, ''Arkivoc'', 2008(i), 128-15 The final step is based on the intramolecular cyclization of an aminal. Reactions * Unsubstituted diaziridines are often directly oxidized (I2/NEt3) to the more stable diazirines. * They can undergo ring expansion reaction with electrophilic reagents like ketenes o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene, the parent hydride from which all other carbene compounds are formally derived. Carbenes are classified as either singlets or triplets, depending upon their electronic structure. Most carbenes are very short lived, although persistent carbenes are known. One well-studied carbene is dichlorocarbene , which can be generated ''in situ'' from chloroform and a strong base. Structures and bonding The two classes of carbenes are singlet and triplet carbenes. Singlet carbenes are spin-paired. In the language of valence bond theory, the molecule adopts an sp2 hybrid structure. Triplet carbenes have two unpaired electrons. Most carbenes have a nonlinear triplet ground state, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brassinosteroid
Brassinosteroids (BRs or less commonly BS) are a class of polyhydroxysteroids that have been recognized as a sixth class of plant hormones and may have utility as an anticancer drug for endocrine-responsive cancers to induce apoptosis and inhibit growth. These brassinosteroids were first explored during the 70s, when Mitchell et al. reported promotion in stem elongation and cell division by the treatment of organic extracts of rapeseed (''Brassica napus'') pollen. Brassinolide was the first isolated brassinosteroid in 1979, when pollen from ''Brassica napus'' was shown to promote stem elongation and cell divisions, and the biologically active molecule was isolated. The yield of brassinosteroids from 230 kg of ''Brassica napus'' pollen was only 10 mg. Since their discovery, over 70 BR compounds have been isolated from plants. Biosynthesis The BR is biosynthesised from campesterol. The biosynthetic pathway was elucidated by Japanese researchers and later shown to be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Heterocycles
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many industrially import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid Precursor Protein Secretase
Secretases are enzymes that "snip" pieces off a longer protein that is embedded in the cell membrane. 300px, Processing of the amyloid-beta precursor protein Among other roles in the cell, secretases act on the amyloid-beta precursor protein (APP) to cleave the protein into three fragments. Sequential cleavage by beta-secretase 1 (BACE) and gamma-secretase (γ-secretase) produces the amyloid-beta peptide fragment that aggregates into clumps called amyloid plaques in the brains affected by Alzheimer's disease. If alpha-secretase (α-secretase) acts on APP first instead of BACE, no amyloid beta is formed because α-secretase recognizes a target protein sequence closer to the cell surface than BACE. The non-pathogenic middle fragment formed by an α/γ cleavage sequence is called P3. Structure The structure of the three secretases varies widely. * The α-secretase gene has not been conclusively identified but is believed to be a metalloproteinase. * BACE is a transmembrane prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-s2
{{Letter-NumberCombDisambig ...
1S or 1s may refer to: * 1s electron, in an atomic orbital * Sabre (computer system)'s IATA code * 1S, a series of Toyota S engines * SSH 1S (WA); see Washington State Route 502, Washington State Route 503 See also * Shilling * Second * Ones (other) *S1 (other) S1, S01, S.I, S-1, S.1, Š-1 or S 1 may refer to: Biology and chemistry * S1 nuclease, an enzyme that digests singled-stranded DNA and RNA * S1: Keep locked up, a safety phrase in chemistry * Primary somatosensory cortex, also known as S1 * Tegaf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
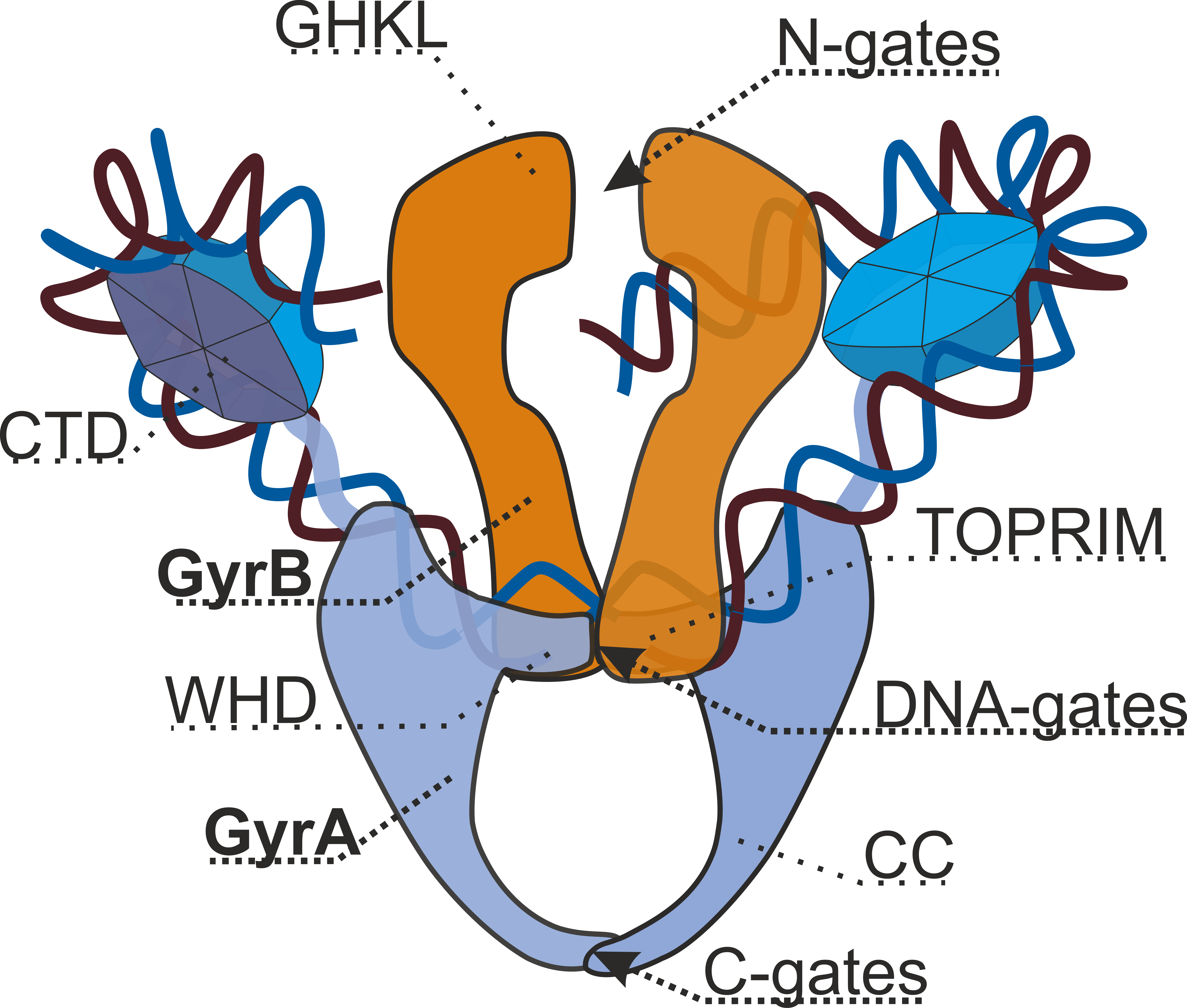
Type II Topoisomerase
Type II topoisomerases are topoisomerases that cut both strands of the DNA helix simultaneously in order to manage DNA tangles and supercoils. They use the hydrolysis of ATP, unlike Type I topoisomerase. In this process, these enzymes change the linking number of circular DNA by ±2. Topoisomerases are ubiquitous enzymes, found in all living organisms. In animals, topoisomerase II is a chemotherapy target. In prokaryotes, gyrase is an antibacterial target. Indeed, these enzymes are of interest for a wide range of effects. Function Type II topoisomerases increase or decrease the linking number of a DNA loop by 2 units, and it promotes chromosome disentanglement. For example, DNA gyrase, a type II topoisomerase observed in '' E. coli'' and most other prokaryotes, introduces negative supercoils and decreases the linking number by 2. Gyrase is also able to remove knots from the bacterial chromosome. Along with gyrase, most prokaryotes also contain a second type IIA topoisomerase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etoposide
Etoposide, sold under the brand name Vepesid among others, is a chemotherapy medication used for the treatments of a number of types of cancer including testicular cancer, lung cancer, lymphoma, leukemia, neuroblastoma, and ovarian cancer. It is also used for hemophagocytic lymphohistiocytosis. It is used by mouth or injection into a vein. Side effects are very common. They can include low blood cell counts, vomiting, loss of appetite, diarrhea, hair loss, and fever. Other severe side effects include allergic reactions and low blood pressure. Use during pregnancy will likely harm the fetus. Etoposide is in the topoisomerase inhibitor family of medication. It is believed to work by damaging DNA. Etoposide was approved for medical use in the United States in 1983. It is on the World Health Organization's List of Essential Medicines. Medical uses Etoposide is used as a form of chemotherapy for cancers such as Kaposi’s sarcoma, Ewing's sarcoma, lung cancer, testicular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GABAA Receptor
The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−). Depending on the membrane potential and the ionic concentration difference, this can result in ionic fluxes across the pore. If the membrane potential is higher than the equilibrium potential (also known as the reversal potential) for chloride ions, when the receptor is activated Cl− will flow into the cell. This causes an inhibitory effect on neurotransmission by diminishing the chance of a successful action potential occurring at the postsynaptic cell. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential (IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100 mV). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propofol
Propofol, marketed as Diprivan, among other names, is a short-acting medication that results in a decreased level of consciousness and a lack of memory for events. Its uses include the starting and maintenance of general anesthesia, sedation for mechanically ventilated adults, and procedural sedation. It is also used for status epilepticus if other medications have not worked. It is given by injection into a vein, and the maximum effect takes about two minutes to occur and typically lasts five to ten minutes. Propofol is also used for medical assistance in dying in Canada. The medication appears to be safe for use during pregnancy but has not been well studied for use in this case. It is not recommended for use during a cesarean section. It is not a pain medication, so opioids such as morphine may also be used; however, whether or not they are always needed is not clear. Propofol is believed to work at least partly via a receptor for GABA. Propofol was discovered i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |