Diazirine on:
[Wikipedia]
[Google]
[Amazon]
In

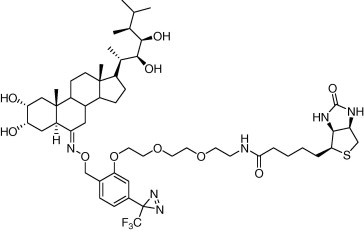 * The discovery of novel non-CB1/CB2
* The discovery of novel non-CB1/CB2
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, a diazirine is an organic molecule
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-cont ...
consisting of a carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
bound to two nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
atoms, which are double-bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwe ...
ed to each other, forming a cyclopropene
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental s ...
-like ring, 3''H''-diazirine (). Diazirines are isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
ic with diazo
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds ...
carbon groups (), and like them can serve as precursors for carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s by loss of a molecule of dinitrogen. For example, irradiation of diazirines with ultraviolet light
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of th ...
leads to carbene insertion into various , , and bonds. Hence, diazirines have grown in popularity as small, photo-reactive, crosslinking Cross-linking may refer to
*Cross-link
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers ca ...
reagents. They are often used in photoaffinity labeling Photoaffinity labeling is a chemoproteomics technique used to attach "labels" to the active site of a large molecule, especially a protein. The "label" attaches to the molecule loosely and reversibly, and has an inactive site which can be converted ...
studies to observe a variety of interactions, including ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
-receptor, ligand-enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
, protein-protein, and protein-nucleic acid
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a pentose, 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nuclei ...
interactions.
Synthesis
A number of methods exist in the literature for the preparation of diazirines, which begin from a variety of reagents.Synthesis from ketones
Generally, synthetic schemes that begin withketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s () involve conversion of the ketone with the desired substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s to diaziridine
A diaziridine is a heterocyclic compound containing two nitrogen atoms in a three-membered ring. Diaziridines can be considered as strained hydrazines. Unlike most amine types of structures, the nitrogen atoms of diaziridines are stereocenter, co ...
s (). These diaziridines are then subsequently oxidized
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
to form the desired diazirines.
Diaziridines can be prepared from ketones by oximation, followed by tosylation
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
(or mesylation
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the group o ...
), and then finally by treatment with ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
(). Generally, oximation reactions are performed by reacting the ketone with hydroxylammonium chloride
Hydroxylammonium chloride is a chemical compound with the formula . It is the hydrochloric acid salt of hydroxylamine (). Hydroxylamine is a biological intermediate in nitrification (biological oxidation of ammonia with oxygen into nitrite) and ...
() under heat in the presence of a base such as pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
. Subsequent tosylation or mesylation of the alpha-substituted oxygen with tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
or mesyl chloride
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula . Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group –, it is frequently abbreviated MsCl in reaction schemes or equations. It ...
in the presence of base yields the tosyl or mesyl oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
. The final treatment of the tosyl or mesyl oxime with ammonia produces the diaziridine.
Diaziridines can be also produced directly by the reaction of ketones with ammonia in the presence of an aminating agent such as a monochloramine
Monochloramine, often called chloramine, is the chemical compound with the formula NH2Cl. Together with dichloramine (NHCl2) and nitrogen trichloride (NCl3), it is one of the three chloramines of ammonia. It is a colorless liquid at its melting ...
or hydroxyl amine O-sulfonic acid.
Diaziridines can be oxidized to diazirines by a number of methods. These include oxidation by chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
-based reagents such as the Jones oxidation
The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of ...
, oxidation by iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
and triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
, oxidation by silver oxide
Silver oxide is the chemical compound with the formula Ag2 O. It is a fine black or dark brown powder that is used to prepare other silver compounds.
Preparation
Silver oxide can be prepared by combining aqueous solutions of silver nitrate and ...
, oxidation by oxalyl chloride, or even electrochemical oxidation on a platinum-titanium anode.
Synthesis by Graham reaction
Diazirines can be alternatively formed in a one-pot process using the Graham reaction, starting fromamidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines includ ...
s. This reaction yields a halogenated
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs ...
diazirine, whose halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
can be displaced by various nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
reagents.
Chemistry
Upon irradiation with UV light, diazirines form reactivecarbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
species. The carbene may exist in the singlet form, in which the two free electrons occupy the same orbital, or the triplet form, with two unpaired electrons in different orbitals.
Triplet vs singlet carbene products
The substituents on the diazirine affect which carbene species is generated upon irradiation and subsequent photolytic cleavage. Diazirine substituents that are electron donating in nature can donate electron density to the empty p-orbital of the carbene that will be formed, and hence can stabilize the singlet state. For example, phenyldiazirine produces phenylcarbene in the singlet carbene state whereas 3-chloro-3- 4-nitrophenyl)methyliazirine or produce the respective triplet carbene products. Electron donating substituents can also encourage photoisomerization to the linear diazo compound, rather than the singlet carbene, and hence these compounds are unfavorable for use in biological assays. On the other hand, in particular show favorable stability and photolytic qualities and are most commonly used in biological applications. Carbenes produced from diazirines are quickly quenched by reaction with water molecules, and hence yields for photoreactive crosslinking assays are often low. Yet, as this feature minimizes unspecific labeling, it is actually an advantage of using diazirines.Use in photoreactive crosslinking
Diazirines are often used as photoreactive crosslinking reagents, as the reactive carbenes they form upon irradiation with UV light can insert into C-H, N-H, and O-H bonds. This results in proximity-dependent labeling of other species with the diazirine containing compound. However, studies have found that diazirines have some pH dependence in labeling preferences, favoring acidic residues such as glutamate. Diazirine variants have been developed to reduce this bias. Diazirines are often preferred to other photoreactive crosslinking reagents due to their smaller size, longer irradiation wavelength, short period of irradiation required, and stability in the presence of various nucleophiles, and in both acidic and basic conditions.Benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and ros ...
s, which form reactive triplet carbonyl species upon irradiation, often require long periods of irradiation which can result in non-specific labeling, and moreover are often inert to various polar solvents. Aryl azides require a low wavelength of irradiation, which can damage the biological macromolecules under investigation.
Examples in receptor labeling studies
Diazirines are widely used in receptor labeling studies. This is because diazirine-containing analogs of various ligands can be synthesized and incubated with their respective receptors, and then subsequently exposed to light to produce reactive carbenes. The carbene will covalently bond to residues in the binding site of the receptor. The carbene compound may include a bioorthogonal tag or handle by which the protein of interest can be isolated. The protein can then be digested and sequenced by mass spectrometry in order to identify which residues the carbene containing ligand is bound to, and hence the identity of the binding site in the receptor. Examples of diazirines used in receptor labeling studies include: * The discovery of abrassinosteroid
Brassinosteroids (BRs or less commonly BS) are a class of polyhydroxysteroids that have been recognized as a sixth class of
plant hormones and may have utility as anticancer drugs for treating endocrine-responsive cancers by inducing apoptosis of ...
receptor for brassinosteroid plant hormones by Kinoshita et al. Researchers used a plant hormone analog with a diazirine crosslinking moiety and a biotin tag for isolation to identity the new receptor. This study led to a number of similar studies conducted with regards to other plant hormones.
 * The discovery of novel non-CB1/CB2
* The discovery of novel non-CB1/CB2 cannabinoid receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system of vertebrates a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cann ...
s using anandamide
Anandamide (ANA), also referred to as ''N''-arachidonoylethanolamine (AEA) is a fatty acid neurotransmitter belonging to the fatty acid derivative group known as N-acylethanolamine (NAE). Anandamide takes its name from the Sanskrit word ''ananda ...
analog probes containing a diazirine group by Balas et al.
* The binding cavity of the hypnotic
A hypnotic (from Ancient Greek, Greek ''Hypnos'', sleep), also known as a somnifacient or soporific, and commonly known as sleeping pills, are a class of psychoactive drugs whose primary function is to sleep induction, induce sleep and to trea ...
agent propofol
Propofol is the active component of an intravenous anesthetic formulation used for induction and maintenance of general anesthesia. It is chemically termed 2,6-diisopropylphenol. The formulation was approved under the brand name Diprivan. Nu ...
in the GABAA receptor using a diazirine containing propofol analog.
Examples in enzyme-substrate studies
In a manner analogous to receptor labeling, diazirine containing compounds that are analogs of natural substrates have also been used to identify binding pockets of enzymes. Examples include: * The synthesis of a diazirine containing analog ofetoposide
Etoposide, sold under the brand name Vepesid among others, is a chemotherapy medication used for the treatments of a number of types of cancer including testicular cancer, lung cancer, lymphoma, leukemia, neuroblastoma, and ovarian cancer. It is ...
, a widely used cancer drug targeting topoisomerase II
Type II topoisomerases are topoisomerases that cut both strands of the DNA helix simultaneously in order to manage DNA tangles and supercoils. They use the hydrolysis of Adenosine triphosphate, ATP, unlike Type I topoisomerase. In this process, t ...
, which holds promise for the identification of the etoposide binding site.
* The discovery that caprolactam-type gamma-secretase inhibitors target the SPP subunit of the gamma-secretase
Gamma secretase is a multi-subunit protease complex, an integral membrane protein, that cleaves single-pass transmembrane proteins at residues within the transmembrane domain. Proteases of this type are known as intramembrane proteases. The most ...
, which has been implicated in Alzheimer's disease.
* The finding that the O-GlcNAc
''O''-GlcNAc (short for ''O''-linked GlcNAc or ''O''-linked β-''N''-acetylglucosamine) is a reversible Enzyme, enzymatic post-translational modification that is found on serine and threonine residues of Cell nucleus, nucleoCytoplasm, cytoplasmi ...
post-translational modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translation (biolog ...
mediates protein-protein interactions such as with 14-3-3 proteins
14-3-3 proteins are a family of conserved regulatory molecules that are expressed in all eukaryotic cells. 14-3-3 proteins have the ability to bind a multitude of functionally diverse signaling proteins, including kinases, phosphatases, and trans ...
.
Examples in nucleic acid studies
Diazirines have been used in photoaffinity labeling experiments involving nucleic acids as well. Examples include: * Incorporation of a diazirine moiety on a nucleoside sugar in a DNA polymer to investigate interactions between the minor groove of DNA and DNA polymerases. * Incorporation of a diazirine moiety on a nucleoside base in a DNA polymer to investigate the mode of DNA repair by proteins. Diazirines have also been used to study protein lipid interactions, for example the interaction of various sphingolipids with proteins in vivo.References
{{reflist Nitrogen heterocycles Photochemistry