|
Diacylglycerol Kinase
Diacylglycerol kinase (DGK or DAGK) is a family of enzymes that catalyzes the conversion of diacylglycerol (DAG) to phosphatidic acid (PA), utilizing ATP as a source of the phosphate. In non-stimulated cells, DGK activity is low, allowing DAG to be used for glycerophospholipid biosynthesis, but on receptor activation of the phosphoinositide pathway, DGK activity increases, driving the conversion of DAG to PA. As both lipids are thought to function as bioactive lipid signaling molecules with distinct cellular targets, DGK therefore occupies an important position, effectively serving as a switch by terminating the signalling of one lipid while simultaneously activating signalling by another. In bacteria, DGK is very small (13 to 15 kDa) membrane protein which seems to contain three transmembrane domains. The best conserved region is a stretch of 12 residues which are located in a cytoplasmic loop between the second and third transmembrane domains. Some Gram-positive bacteria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diglyceride
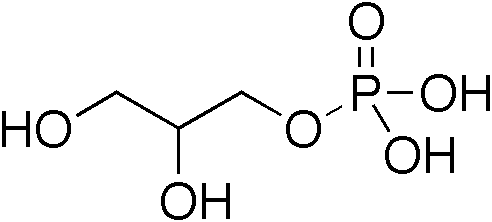
A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil (particularly 1,3-DAG) has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009. Production Diglycerides are a minor component of many seed oils and are normally present at ~1–6%; or in the case of cottonseed oil as much as 10%. Industrial production is primarily achieved by a glycerolysis reaction between triglycerides and glycerol. The raw materials for this may be either vegetable oil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipotechoic Acid
Lipoteichoic acid (LTA) is a major constituent of the cell wall of gram-positive bacteria. These organisms have an inner (or cytoplasmic) membrane and, external to it, a thick (up to 80 nanometer) peptidoglycan layer. The structure of LTA varies between the different species of gram-positive bacteria and may contain long chains of ribitol or glycerol phosphate. LTA is anchored to the cell membrane via a diacylglycerol. It acts as regulator of autolytic wall enzymes ( muramidases). It has antigenic properties being able to stimulate specific immune response. LTA may bind to target cells non-specifically through membrane phospholipids, or specifically to CD14 and to Toll-like receptors. Binding to TLR-2 has shown to induce NF-κB expression(a central transcription factor), elevating expression of both pro- and anti-apoptotic genes. Its activation also induces mitogen-activated protein kinases (MAPK) activation along with phosphoinositide 3-kinase activation. Studies LTA's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
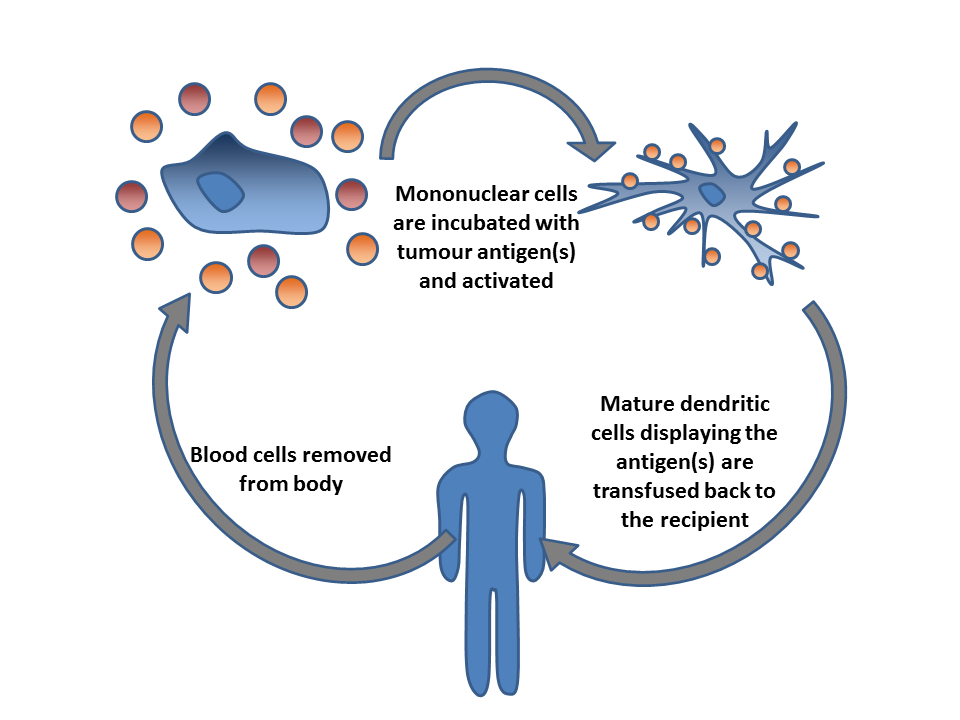
Cancer Immunotherapy
Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the basic research, fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology. Cancer immunotherapy exploits the fact that cancer cells often have tumor antigens, molecules on their surface that can bind to antibody proteins or T-cell receptors, triggering an immune system response. The tumor antigens are often proteins or other macromolecules (e.g., carbohydrates). Normal antibodies bind to external pathogens, but the modified immunotherapy antibodies bind to the tumor antigens marking and identifying the cancer cells for the immune system to inhibit or kill. The clinical success of cancer immunotherapy is highly variable between different forms of cancer; for instance, certain subtypes of gastric cancer react well to the approach whereas immunothera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Programmed Cell Death Protein 1
Programmed cell death protein 1 (PD-1), (CD279 cluster of differentiation 279). PD-1 is a protein encoded in humans by the ''PDCD1'' gene. PD-1 is a cell surface receptor on T cells and B cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. This prevents autoimmune diseases, but it can also prevent the immune system from killing cancer cells. PD-1 is an immune checkpoint and guards against autoimmunity through two mechanisms. First, it promotes apoptosis (programmed cell death) of antigen-specific T-cells in lymph nodes. Second, it reduces apoptosis in regulatory T cells (anti-inflammatory, suppressive T cells). PD-1 inhibitors, a new class of drugs that block PD-1, activate the immune system to attack tumors and are used to treat certain types of cancer. PD-1 is a cell surface receptor that belongs to the immunoglobulin sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin-2
Interleukin-2 (IL-2) is an interleukin, which is a type of cytokine signaling molecule forming part of the immune system. It is a 15.5–16 kDa protein that regulates the activities of white blood cells (leukocytes, often lymphocytes) that are responsible for immunity. IL-2 is part of the body's natural response to microbial infection, and in discriminating between foreign ("non-self") and "self". IL-2 mediates its effects by binding to IL-2 receptors, which are expressed by lymphocytes. The major sources of IL-2 are activated CD4+ T cells and activated CD8+ T cells. Put shortly the function of IL-2 is to stimulate the growth of helper, cytotoxic and regulatory T cells. IL-2 receptor IL-2 is a member of a specific family of cytokines, each member of which has a four alpha helix bundle; this cytokine family also includes IL-4, IL-7, IL-9, IL-15 and IL-21. IL-2 signals through a IL-2 receptor, a complex consisting of three chains, termed alpha ( CD25), be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PDZ Domain
The PDZ domain is a common structural domain of 80-90 Amino acid, amino-acids found in the Signal transduction, signaling proteins of bacteria, yeast, plants, viruses and animals. Proteins containing PDZ domains play a key role in anchoring receptor proteins in the membrane to cytoskeletal components. Proteins with these domains help hold together and organize signaling complexes at cellular membranes. These domains play a key role in the formation and function of signal transduction complexes. PDZ domains also play a highly significant role in the anchoring of cell surface receptors (such as Cftr and FZD7) to the actin cytoskeleton via mediators like NHERF and ezrin. ''PDZ'' is an initialism combining the first letters of the first three proteins discovered to share the domain — DLG4, post synaptic density protein (PSD95), DLG1, Drosophila disc large tumor suppressor (Dlg1), and Tight junction protein 1, zonula occludens-1 protein (zo-1). PDZ domains have previously been referr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Localisation Signal
A nuclear localization signal ''or'' sequence (NLS) is an amino acid sequence that 'tags' a protein for import into the cell nucleus by nuclear transport. Typically, this signal consists of one or more short sequences of positively charged lysines or arginines exposed on the protein surface. Different nuclear localized proteins may share the same NLS. An NLS has the opposite function of a nuclear export signal (NES), which targets proteins out of the nucleus. Types Classical These types of NLSs can be further classified as either monopartite or bipartite. The major structural differences between the two are that the two basic amino acid clusters in bipartite NLSs are separated by a relatively short spacer sequence (hence bipartite - 2 parts), while monopartite NLSs are not. The first NLS to be discovered was the sequence PKKKRKV in the SV40 Large T-antigen (a monopartite NLS). The NLS of nucleoplasmin, KR AATKKAGQAKKK, is the prototype of the ubiquitous bipartite signal: two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
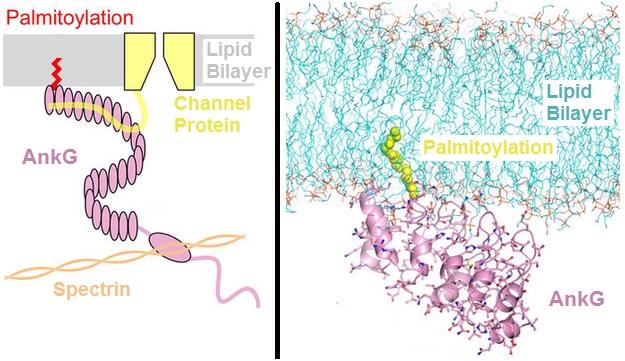
Ankyrin
Ankyrins are a family of proteins that mediate the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton. Ankyrins have binding sites for the beta subunit of spectrin and at least 12 families of integral membrane proteins. This linkage is required to maintain the integrity of the plasma membranes and to anchor specific ion channels, ion exchangers and ion transporters in the plasma membrane. The name is derived from the Greek language, Greek word wikt:ἄγκυρα, ἄγκυρα (''ankyra'') for "anchor". Structure Ankyrins contain four functional Structural domain, domains: an N-terminal domain that contains 24 tandem ankyrin repeats, a central domain that binds to spectrin, a death domain that binds to proteins involved in apoptosis, and a C-terminal regulatory domain that is highly variable between different ankyrin proteins. Membrane protein recognition The 24 tandem ankyrin repeats are responsible for the recognition of a wide range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MARCKS
Marcks is a surname. Notable people with the surname include: * Erich Marcks (1891–1944), German general * Gerhard Marcks (1889–1981), German sculptor * Greg Marcks (born 1976), American director, writer and actor * Megan Marcks (born 1972), Australian rower * Werner Marcks (1896–1967), German general See also * 10778 Marcks, a main-belt asteroid * Marks (other) * Marks (surname) * Marx (other) * Marx (surname) The surname Marx is a Germanic surname, believed to originate with Mark the Evangelist and the Roman praenomen Marcus, the latter deriving from the god Mars. The similarly-spelled Marks may share etymology with march (territory), especially near Wa ... {{surname, Marcks Surnames from given names ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arachidonate
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega−6 fatty acid 20:4(ω−6), or 20:4(5,8,11,14). It is a precursor in the formation of leukotrienes, prostaglandins, and thromboxanes. Together with omega−3 fatty acids and other omega−6 fatty acids, arachidonic acid provides energy for body functions, contributes to cell membrane structure, and participates in the synthesis of eicosanoids, which have numerous roles in physiology as signaling molecules. Its name derives from the ancient Greek neologism ''arachis'' 'peanut', although peanut oil does not contain any arachidonic acid. Arachidonate is the name of the derived carboxylate anion (conjugate base of the acid), salts, and some esters. Chemistry In chemical structure, arachidonic acid is a carboxylic acid with a 20-carbon chain and four '' cis''-double bonds; the first double bond is located at the sixth carbon from the omega end. Some chemistry sources define 'arachidonic acid' to designate any o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleckstrin Homology
Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosphatidylinositol lipids within biological membranes (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate), and proteins such as the G beta-gamma complex, βγ-subunits of heterotrimeric G proteins, and protein kinase C. Through these interactions, PH domains play a role in recruiting proteins to different cell membrane, membranes, thus targeting them to appropriate Cell compartment, cellular compartments or enabling them to interact with other components of the signal transduction pathways. Lipid binding specificity Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e.g., some bind phosphatidylinositol (4,5)-bisphosphate but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recoverin
Recoverin is a 23 kilodalton (kDa) neuronal calcium-binding protein that is primarily detected in the photoreceptor cells of the eye. It plays a key role in the inhibition of rhodopsin kinase, a molecule which regulates the phosphorylation of rhodopsin. A reduction in this inhibition helps regulate sensory adaptation in the retina, since the light-dependent channel closure in photoreceptors causes calcium levels to decrease, which relieves the inhibition of rhodopsin kinase by calcium-bound recoverin, leading to a more rapid inactivation of metarhodopsin II (activated form of rhodopsin). Structure Recoverin structure consists of four EF-hand motifs arranged in a compact array, which contrasts with the dumbbell shape of other calcium-binding proteins like calmodulin and troponin C Troponin C is a protein which is part of the troponin complex. It contains four calcium-binding EF hands, although different isoforms may have fewer than four functional calcium-binding subdomai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |