|
Dehydrogenation Of Amine-boranes
Dehydrogenation of amine-boranes or dehydrocoupling of amine-boranes is a chemical process in main group and organometallic chemistry wherein dihydrogen is released by the coupling of two or more amine-borane adducts. This process is of due to the potential of using amine-boranes for hydrogen storage. Catalysis Many metal complexes catalyze the dehydrogenation of amine-borane (AB). Catalysis in the absence of metals has also been observed.Staubitz, A.; Robertson, A. P. M.; Manners, I., "Ammonia-Borane and Related Compounds as Dihydrogen Sources", Chemical Reviews 2010, volume 110, pp. 4079–4124.. Pathways The dehydrogenation of AB would in principle afford (H2BNH2)n and (HBNH)n. The monomers (n = 1) are highly unstable with respect to oligomerization. Metal carbonyl catalysts Group 6 metal carbonyls upon photolytic activation catalyze dehydrogenation of AB. Secondary amine-boranes dehydrogenate to form cyclic dimers, or monomeric aminoboranes in the case of more bulky group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Process
In a scientific sense, a chemical process is a method or means of somehow changing one or more chemicals or chemical compounds. Such a chemical process can occur by itself or be caused by an outside force, and involves a chemical reaction of some sort. In an "engineering" sense, a chemical process is a method intended to be used in manufacturing or on an industrial scale (see Industrial process) to change the composition of chemical(s) or material(s), usually using technology similar or related to that used in chemical plants or the chemical industry. Neither of these definitions are exact in the sense that one can always tell definitively what is a chemical process and what is not; they are practical definitions. There is also significant overlap in these two definition variations. Because of the inexactness of the definition, chemists and other scientists use the term "chemical process" only in a general sense or in the engineering sense. However, in the "process (engine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterogeneous Catalysis
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures (e.g. oil and water), or anywhere an interface is present. Heterogeneous catalysis typically involves solid phase catalysts and gas phase reactants. In this case, there is a cycle of molecular adsorption, reaction, and desorption occurring at the catalyst surface. Thermodynamics, mass transfer, and heat transfer influence the rate (kinetics) of reaction. Heterogeneous catalysis is very important because it enables faster, large-scale production and the selective product formation. Approximately 35% of the world's GDP is influenced by catalysis. The production of 90% of chemicals (by volume) is assisted by solid catalysts. The chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Markovnikov's Rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870. Explanation The rule states that with the addition of a protic acid HX or other polar reagent to an asymmetric alkene, the acid hydrogen (H) or electropositive part gets attached to the carbon with more hydrogen substituents, and the halide (X) group or electronegative part gets attached to the carbon with more alkyl substituents. This is in contrast to Markovnikov's original definition, in which the rule is stated that the X component is added to the carbon with the fewest hydrogen atoms while the hydrogen atom is added to the carbon with the greatest number of hydrogen atoms. The same is true when an alkene reacts with water in an addition reaction to form an alcohol which involve formation of carbocations. The hydroxyl group (OH) bonds to the carbon that has the greater number of carbon–c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For this reason borazine is sometimes referred to as “inorganic benzene”. Like benzene, borazine is a colourless liquid with an aromatic smell. Synthesis The compound was reported in 1926 by the chemists Alfred Stock and Erich Pohland by a reaction of diborane with ammonia. Borazine can be synthesized by treating diborane and ammonia in a 1:2 ratio at 250–300 °C with a conversion of 50%. :3 B2H6 + 6 NH3 → 2 B3H6N3 + 12 H2 An alternative more efficient route begins with sodium borohydride and ammonium sulfate: :6 NaBH4 + 3 (NH4)2SO4 → 2 B3N3H6 + 3 Na2SO4 + 18 H2 In a two-step process to borazine, boron trichloride is first converted to trichloroborazine: :3 BCl3 + 3 NH4Cl → Cl3B3H3N3 + 9 HCl The B-Cl bonds are subseq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia Borane
Ammonia borane (also systematically named amminetrihydridoboron), also called borazane, is the chemical compound with the formula H3NBH3. The colourless or white solid is the simplest molecular boron-nitrogen-hydride compound. It has attracted attention as a source of hydrogen fuel, but is otherwise primarily of academic interest. Synthesis Reaction of diborane with ammonia mainly gives the diammoniate salt 2B(NH3)2sup>+ (BH4)−. Ammonia borane is the main product when an adduct of borane is employed in place of diborane: :BH3( THF) + NH3 → BH3NH3 + THF Properties and structure The molecule adopts a structure similar to that of ethane, with which it is isoelectronic. The B−N distance is 1.58(2) Å. The B−H and N−H distances are 1.15 and 0.96 Å, respectively. Its similarity to ethane is tenuous since ammonia borane is a solid and ethane is a gas: their melting points differing by 284 °C. This difference is consistent with the highly polar na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallocenes
A metallocene is a compound typically consisting of two cyclopentadienyl anions (, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to p2ZrCH3sup>+ catalyze olefin polymerization. Some metallocenes consist of metal plus two cyclooctatetraenide anions (, abbreviated cot2−), namely the lanthanocenes and the actinocenes ( uranocene and others). Metallocenes are a subset of a broader class of compounds called sandwich compounds. In the structure shown at right, the two pentagons are the cyclopentadienyl anions with circles inside them indicating they are aromatically stabilized. Here they are shown in a staggered conformation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilkinson's Catalyst
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula hCl(PPh3)3(Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use. Historically, Wilkinson's catalyst has been a paradigm in catalytic studies leading to several advances in the field such as the implementation of some of the first heteronuclear magnetic resonance studies for its structural elucidation in solution (31P), parahydrogen-induced polarization spectroscopy to determine the nature of transient reactive species, or one of the first detailed kinetic investigation by Halpern to elucidate the mechanism. Furthermore, the catalytic and organome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
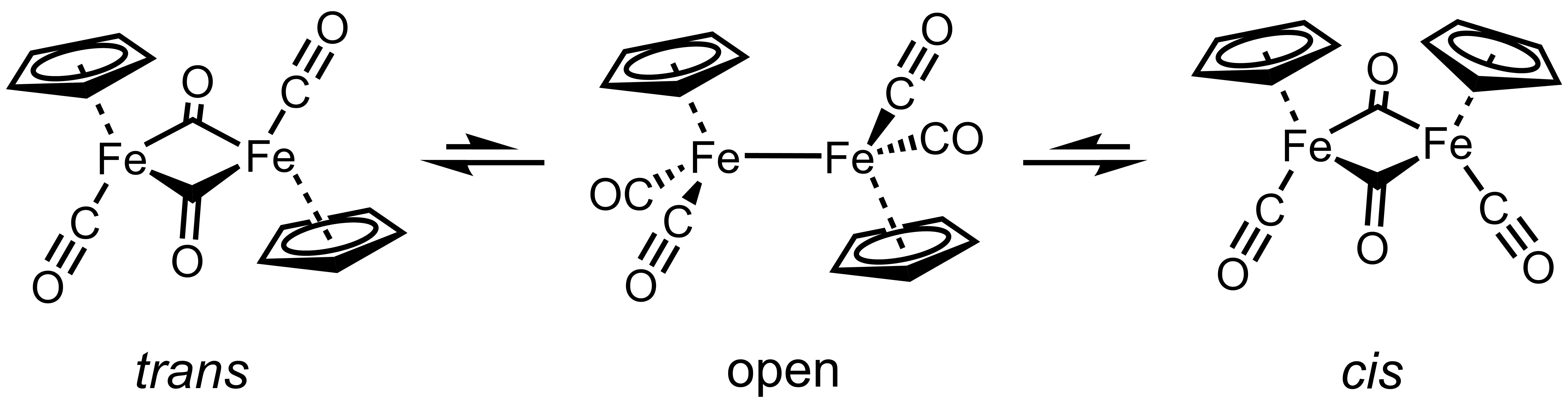
Cyclopentadienyliron Dicarbonyl Dimer
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula ''η''5-C5H5)Fe(CO)2sub>2, often abbreviated to Cp2Fe2(CO)4, pFe(CO)2sub>2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives (described below). Structure In solution, Cp2Fe2(CO)4 can be considered a dimeric half-sandwich complex. It exists in three isomeric forms: ''cis'', ''trans'', and an unbridged, open form. These isomeric forms are distinguished by the position of the ligands. The ''cis'' and ''trans'' isomers differ in the relative position of C5H5 (Cp) ligands. The ''cis'' and ''trans'' is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Main Group
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arranged in the periodic table of the elements. The main group includes the elements (except hydrogen, which is sometimes not included) in groups 1 and 2 ( s-block), and groups 13 to 18 ( p-block). The s-block elements are primarily characterised by one main oxidation state, and the p-block elements, when they have multiple oxidation states, often have common oxidation states separated by two units. Main-group elements (with some of the lighter transition metals) are the most abundant elements on Earth, in the Solar System, and in the universe. Group 12 elements are often considered to be transition metals; however, zinc (Zn), cadmium (Cd), and mercury (Hg) share some properties of both groups, and some scientists believe they sho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes. Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of oxygen. Nomenclature and terminology The nomenclature of the metal carbonyls depends on the charge of the complex, the number and type of central atoms, and the number and type of ligands and their binding modes. They occur as neutral complexes, as positively-charged metal carbonyl cations or as negatively charged metal carbonylates. The carbon monoxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |