Cyclopentadienyliron Dicarbonyl Dimer on:
[Wikipedia]
[Google]
[Amazon]
Cyclopentadienyliron dicarbonyl dimer is an
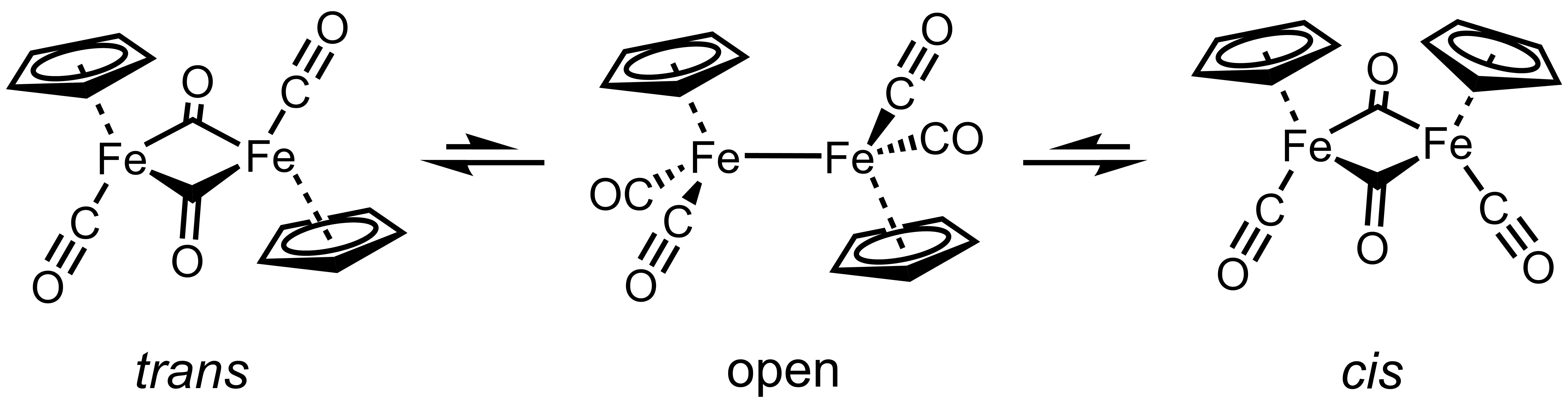 In addition, the terminal and bridging carbonyls are known to undergo exchange: the ''trans'' isomer can undergo bridging–terminal CO ligand exchange through the open isomer, or through a twisting motion without going through the open form. In contrast, the bridging and terminal CO ligands of the ''cis'' isomer can only exchange via the open isomer.
In solution, the ''cis'', ''trans'', and open isomers interconvert rapidly at room temperature, making the molecular structure fluxional. The fluxional process for cyclopentadienyliron dicarbonyl dimer is faster than the NMR time scale, so that only an averaged, single Cp signal is observed in the 1H NMR spectrum at 25 °C. Likewise, the 13C NMR spectrum exhibits one sharp CO signal above −10 °C, while the Cp signal sharpens to one peak above 60 °C. NMR studies indicate that the ''cis'' isomer is slightly more abundant than the ''trans'' isomer at room temperature, while the amount of the open form is small. The fluxional process is not fast enough to produce averaging in the IR spectrum. Thus, three absorptions are seen for each isomer. The bridging CO ligands appear at around 1780 cm−1 whereas the terminal CO ligands are observed at around 1980 cm−1. The averaged structure of these isomers of Cp2Fe2(CO)4 results in a dipole moment of 3.1 D in
In addition, the terminal and bridging carbonyls are known to undergo exchange: the ''trans'' isomer can undergo bridging–terminal CO ligand exchange through the open isomer, or through a twisting motion without going through the open form. In contrast, the bridging and terminal CO ligands of the ''cis'' isomer can only exchange via the open isomer.
In solution, the ''cis'', ''trans'', and open isomers interconvert rapidly at room temperature, making the molecular structure fluxional. The fluxional process for cyclopentadienyliron dicarbonyl dimer is faster than the NMR time scale, so that only an averaged, single Cp signal is observed in the 1H NMR spectrum at 25 °C. Likewise, the 13C NMR spectrum exhibits one sharp CO signal above −10 °C, while the Cp signal sharpens to one peak above 60 °C. NMR studies indicate that the ''cis'' isomer is slightly more abundant than the ''trans'' isomer at room temperature, while the amount of the open form is small. The fluxional process is not fast enough to produce averaging in the IR spectrum. Thus, three absorptions are seen for each isomer. The bridging CO ligands appear at around 1780 cm−1 whereas the terminal CO ligands are observed at around 1980 cm−1. The averaged structure of these isomers of Cp2Fe2(CO)4 results in a dipole moment of 3.1 D in
 Fp(alkene)+ and Fp(alkyne)+ π-complexes are also quite acidic at the allylic and propargylic positions, respectively, and can be quantitatively deprotonated with amine bases like Et3N to give neutral Fp–allyl and Fp–allenyl σ-complexes (eqn 1, shown for alkene complex).
Fp(alkene)+ and Fp(alkyne)+ π-complexes are also quite acidic at the allylic and propargylic positions, respectively, and can be quantitatively deprotonated with amine bases like Et3N to give neutral Fp–allyl and Fp–allenyl σ-complexes (eqn 1, shown for alkene complex).
 Fp–allyl and Fp–allenyl react with cationic electrophiles E (such as Me3O+,
Fp–allyl and Fp–allenyl react with cationic electrophiles E (such as Me3O+,
organometallic compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
with the formula ''η''5-C5H5)Fe(CO)2sub>2, often abbreviated to Cp2Fe2(CO)4, pFe(CO)2sub>2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
and pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weak ...
, but less soluble in carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a n ...
and carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula and structure . It is also considered as the anhydride of thiocarbonic acid. It is a colorless, flammable, neurotoxic liquid that is used as ...
. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives.
Structure
In solution, Cp2Fe2(CO)4 can be considered a dimeric half-sandwich complex. It exists in three isomeric forms: ''cis'', ''trans'', and an unbridged, open form. These isomers are distinguished by the position of the ligands. The ''cis'' and ''trans'' isomers differ in the relative position of C5H5 (Cp) ligands. The ''cis'' and ''trans'' isomers have the formulation ''η''5-C5H5)Fe(CO)(''μ''-CO)sub>2, that is, two CO ligands are terminal whereas the other two CO ligands bridge between the iron atoms. The ''cis'' and ''trans'' isomers interconvert via the open isomer, which has no bridging ligands between iron atoms. Instead, it is formulated as (''η''5-C5H5)(OC)2Fe−Fe(CO)2(''η''5-C5H5) — the metals are held together by an iron–iron bond. At equilibrium, the ''cis'' and ''trans'' isomers are predominant. : In addition, the terminal and bridging carbonyls are known to undergo exchange: the ''trans'' isomer can undergo bridging–terminal CO ligand exchange through the open isomer, or through a twisting motion without going through the open form. In contrast, the bridging and terminal CO ligands of the ''cis'' isomer can only exchange via the open isomer.
In solution, the ''cis'', ''trans'', and open isomers interconvert rapidly at room temperature, making the molecular structure fluxional. The fluxional process for cyclopentadienyliron dicarbonyl dimer is faster than the NMR time scale, so that only an averaged, single Cp signal is observed in the 1H NMR spectrum at 25 °C. Likewise, the 13C NMR spectrum exhibits one sharp CO signal above −10 °C, while the Cp signal sharpens to one peak above 60 °C. NMR studies indicate that the ''cis'' isomer is slightly more abundant than the ''trans'' isomer at room temperature, while the amount of the open form is small. The fluxional process is not fast enough to produce averaging in the IR spectrum. Thus, three absorptions are seen for each isomer. The bridging CO ligands appear at around 1780 cm−1 whereas the terminal CO ligands are observed at around 1980 cm−1. The averaged structure of these isomers of Cp2Fe2(CO)4 results in a dipole moment of 3.1 D in
In addition, the terminal and bridging carbonyls are known to undergo exchange: the ''trans'' isomer can undergo bridging–terminal CO ligand exchange through the open isomer, or through a twisting motion without going through the open form. In contrast, the bridging and terminal CO ligands of the ''cis'' isomer can only exchange via the open isomer.
In solution, the ''cis'', ''trans'', and open isomers interconvert rapidly at room temperature, making the molecular structure fluxional. The fluxional process for cyclopentadienyliron dicarbonyl dimer is faster than the NMR time scale, so that only an averaged, single Cp signal is observed in the 1H NMR spectrum at 25 °C. Likewise, the 13C NMR spectrum exhibits one sharp CO signal above −10 °C, while the Cp signal sharpens to one peak above 60 °C. NMR studies indicate that the ''cis'' isomer is slightly more abundant than the ''trans'' isomer at room temperature, while the amount of the open form is small. The fluxional process is not fast enough to produce averaging in the IR spectrum. Thus, three absorptions are seen for each isomer. The bridging CO ligands appear at around 1780 cm−1 whereas the terminal CO ligands are observed at around 1980 cm−1. The averaged structure of these isomers of Cp2Fe2(CO)4 results in a dipole moment of 3.1 D in benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
.
The solid-state molecular structure of both ''cis'' and ''trans'' isomers have been analyzed by X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
and neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of Neutron temperature, thermal or ...
. The Fe–Fe separation and the Fe–C bond lengths are the same in the Fe2C2 rhomboids, an exactly planar Fe2C2 four-membered ring in the ''trans'' isomer versus a folded rhomboid in ''cis'' with an angle of 164°, and significant distortions in the Cp ring of the ''trans'' isomer reflecting different Cp orbital populations. Although older textbooks show the two iron atoms bonded to each other, theoretical analyses indicate the absence of a direct Fe–Fe bond. This view is consistent with computations and X-ray crystallographic data that indicate a lack of significant electron density between the iron atoms. However, Labinger offers a dissenting view, based primarily on chemical reactivity and spectroscopic data, arguing that electron density is not necessarily the best indication of the presence of a chemical bond. Moreover, without an Fe–Fe bond, the bridging carbonyls must be formally treated as an μ-X2 ligand and μ-L ligand in order for the iron centers to satisfy the 18-electron rule. This formalism is argued to give misleading implications with respect to the chemical and spectroscopic behavior of the carbonyl groups.Synthesis
Cp2Fe2(CO)4 was first prepared in 1955 using the same method employed today: the reaction ofiron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor t ...
and dicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula . At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with ...
.
:2 Fe(CO)5 + C10H12 → (''η''5-C5H5)2Fe2(CO)4 + 6 CO + H2
In this preparation, dicyclopentadiene cracks to give cyclopentadiene, which reacts with Fe(CO)5 with loss of CO. Thereafter, the pathways for the photochemical and thermal routes differ subtly but both entail formation of a hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
intermediate. The method is used in the teaching laboratory.
Reactions
Although of no major commercial value, Fp2 was a workhorse inorganometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
because it is inexpensive and FpX derivatives are rugged (X = halide, organyl).
"Fp−" (FpNa and FpK)
Reductive cleavage of pFe(CO)2sub>2 produces alkali metal derivatives. A typical reductant is sodium metal orsodium amalgam
Sodium amalgam, with the common formula Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. ...
; NaK alloy, potassium graphite (KC8), and alkali metal trialkylborohydrides. pFe(CO)2a is readily alkylated, acylated, or metalated by treatment with an appropriate electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
. It is an excellent SN2 nucleophile, being one to two orders of magnitude more nucleophilic than thiophenolate, PhS– when reacted with primary and secondary alkyl bromides.
: pFe(CO)2sub>2 + 2 Na → 2 CpFe(CO)2Na
: pFe(CO)2sub>2 + 2 KBH(C2H5)3 → 2 CpFe(CO)2K + H2 + 2 B(C2H5)3
Treatment of NaFp with an methyl iodide produces the methyl derivative:
:
Fp2 can also be cleaved electrochemically.
FpX (X = Cl, Br, I)
Halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s oxidatively cleave pFe(CO)2sub>2 to give the Fe(II) species FpX (X = Cl, Br, I):
: pFe(CO)2sub>2 + X2 → 2 CpFe(CO)2X
One example is cyclopentadienyliron dicarbonyl iodide.
Fp(''η''2-alkene)+, Fp(''η''2-alkyne)+ and other "Fp+"
In the presence of halide anion acceptors such as aluminium bromide orsilver tetrafluoroborate
Silver tetrafluoroborate is an inorganic compound with the molecular formula AgBF4. It is a white solid, although commercial samples often are gray, that dissolves in polar organic solvents as well as water.
Preparation
Silver tetrafluoroborate ca ...
, FpX compounds (X = halide) react with alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
, alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
, or neutral labile ligands (such as ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s and nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or pr ...
s) to afford Fp+ complexes. In another approach, salts of p(isobutene)sup>+ are readily obtained by reaction of NaFp with methallyl chloride followed by protonolysis. This complex is a convenient and general precursor to other cationic Fp–alkene and Fp–alkyne complexes. The exchange process is facilitated by the loss of gaseous and bulky isobutene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Productio ...
. Generally, less substituted alkenes bind more strongly and can displace more hindered alkene ligands. Alkene and alkyne complexes can also be prepared by heating a cationic ether or aqua complex, for example , with the alkene or alkyne. complexes can also be prepared by treatment of FpMe with HBF4· Et2O in CH2Cl2 at −78 °C, followed by addition of L.
:
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
–Fp complexes can also be prepared from Fp anion indirectly. Thus, hydride abstraction from Fp–alkyl compounds using triphenylmethyl hexafluorophosphate affords p(α-alkene)sup>+ complexes.
:FpNa + RCH2CH2I → FpCH2CH2R + NaI
:FpCH2CH2R + Ph3CPF6 → + Ph3CH
Reaction of NaFp with an epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
followed by acid-promoted dehydration also affords alkene complexes. Fp(alkene)+ are stable with respect to bromination
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs ...
, hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
, and acetoxymercuration, but the alkene is easily released with sodium iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions ...
in acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
or by warming with acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
.
:
The alkene ligand in these cations is activated toward attack by nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s, opening the way to a number of carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed between on ...
-forming reactions. Nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
s usually occur at the more substituted carbon. This regiochemistry
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
is attributed to the greater positive charge density
In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter ρ) is the quantity of charge per unit volume, measured in the SI system in co ...
at this position. The regiocontrol is often modest. The addition of the nucleophile is completely stereoselective, occurring ''anti'' to the Fp group. Analogous Fp(alkyne)+ complexes are also reported to undergo nucleophilic addition reactions by various carbon, nitrogen, and oxygen nucleophiles.
: Fp(alkene)+ and Fp(alkyne)+ π-complexes are also quite acidic at the allylic and propargylic positions, respectively, and can be quantitatively deprotonated with amine bases like Et3N to give neutral Fp–allyl and Fp–allenyl σ-complexes (eqn 1, shown for alkene complex).
Fp(alkene)+ and Fp(alkyne)+ π-complexes are also quite acidic at the allylic and propargylic positions, respectively, and can be quantitatively deprotonated with amine bases like Et3N to give neutral Fp–allyl and Fp–allenyl σ-complexes (eqn 1, shown for alkene complex).
 Fp–allyl and Fp–allenyl react with cationic electrophiles E (such as Me3O+,
Fp–allyl and Fp–allenyl react with cationic electrophiles E (such as Me3O+, carbocations
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
, oxocarbenium ions) to generate allylic and propargylic functionalization products, respectively (eqn 2, shown for allyliron). The related complex p*Fe(CO)2(thf)sup>+ F4sup>− (Cp* = C5Me5) has been shown to catalyze propargylic, allylic, and allenic C−H functionalization by combining the deprotonation and electrophilic functionalization processes described above with facile exchange of the unsaturated hydrocarbon bound to the cationic iron center.
η2-Allenyl complexes of Fp+ and substituted cyclopentadienyliron dicarbonyl cations have also been characterized, with X-ray crystallographic analysis showing substantial bending at the central allenic carbon (bond angle < 150°).
Fp-based cyclopropanation reagents
Fp-based reagents have been developed forcyclopropanation
In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane () Ring (chemistry), rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroid insectic ...
s. The key reagent is prepared from FpNa with a thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have ...
and methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h ...
, and has a good shelf-life, in contrast to typical Simmons-Smith intermediates and diazoalkane
In organic chemistry, the diazo group is an organic moiety (chemistry), moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are cal ...
s.
:FpNa + ClCH2SCH3 → FpCH2SCH3 + NaCl
:FpCH2SCH3 + CH3I + NaBF4 → FpCH2S(CH3)2]BF4 + NaI
Use of pCH2S(CH3)2F4 does not require specialized conditions.
: + (Ph)2C=CH2 → 1,1-diphenylcyclopropane + …
Iron(III) chloride
Iron(III) chloride describes the inorganic compounds with the formula (H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated f ...
is added to destroy any byproduct.
Precursors to , like FpCH2OMe which is converted to the iron carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
upon protonation, have also been used as cyclopropanation reagents.
Photochemical reaction
Fp2 exhibitsphotochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 Nanometre, nm), visible ligh ...
. For example, upon UV irradiation at 350 nm, it is reduced by benzylnicotinamide, 1-benzyl-1,4-dihydronicotinamide dimer, also known as (BNA)2.
:
References
{{iron compounds Organoiron compounds Carbonyl complexes Cyclopentadienyl complexes Dimers (chemistry) Half sandwich compounds