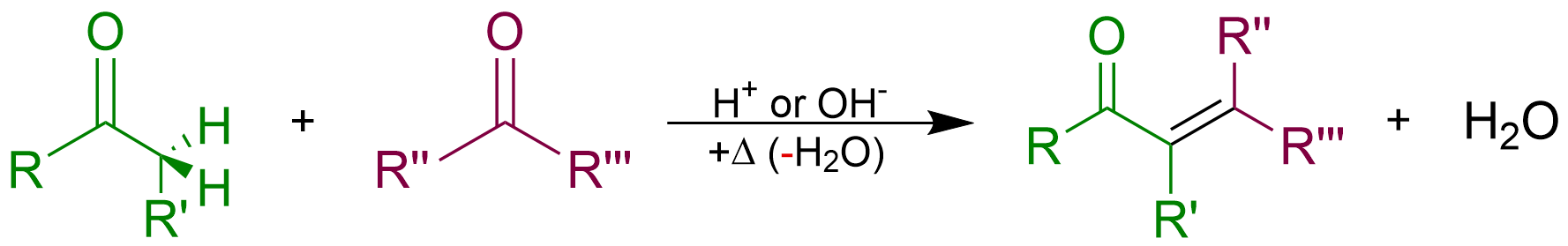|
Cyclamen Aldehyde
Cyclamen aldehyde is a fragrance molecule which has been used in soaps, detergents, lotions, and perfumes since the 1920s. It was granted generally recognized as safe (GRAS) status by the Flavor and Extract Manufacturers Association (FEMA) in 1965 and is approved by the Food and Drug Administration for food use in the United States. The Council of Europe (1970) included cyclamen aldehyde in the list of admissible artificial flavoring substances, at a level of 1 ppm. Synthesis Cyclamen aldehyde is not naturally occurring and is prepared by the crossed-aldol condensation of cuminaldehyde and propionaldehyde followed by hydrogenation Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ... in the presence of a catalyst. References {{reflist Aldehydes Perfume ingredients ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generally Recognized As Safe
Generally recognized as safe (GRAS) is a United States Food and Drug Administration (FDA) designation that a chemical or substance added to food is considered safe by experts under the conditions of its intended use. An ingredient with a GRAS designation is exempted from the usual Federal Food, Drug, and Cosmetic Act (FFDCA) food additive tolerance requirements. The concept of food additives being "generally recognized as safe" was first described in the Food Additives Amendment of 1958, and all additives introduced after this time had to be evaluated by new standards. Some examples of substances recognized as GRAS include ascorbic acid (vitamin C), citric acid, and salt, which are all commonly used in food preservation and flavoring. The FDA list of GRAS notices is updated approximately each month, as of 2021. History On 6 September 1958, the Food Additives Amendment of 1958 was signed into law, with a list of 700 food substances that were exempt from the then-new requirement ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavor And Extract Manufacturers Association
The Flavor and Extract Manufacturers Association (FEMA) is a food industry trade group based in the United States. FEMA was founded in 1909 by several flavor firms in response to the passage of the Pure Food and Drug Act of 1906. Founding members were McCormick & Company, Ulman Driefus & Company, Jones Brothers, Blanke Baer Chemical Company, Frank Tea & Spice Company, Foote & Jenkes, Sherer Gillett Company, and C.F. Sauer Company. Since its founding, FEMA has played instrumental roles in creating a program to assess the safety and "generally recognized as safe" status of flavor ingredients, advocating for policies that positively impact the food and flavor industry, and in representing its members' interests during the creation of the Food Additives Amendment of 1958, an amendment to the United States' Food, Drugs, and Cosmetic Act of 1938. FEMA maintains a Flavor Ingredient Library, a list of all flavoring ingredients allowed in the United States. Critics of FEMA have said th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction equation is as follows (where the Rs can be H) Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or aldol (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, the first step is formally an addition reaction rather than a condensation reaction bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cuminaldehyde
Cuminaldehyde (4-isopropylbenzaldehyde) is a natural organic compound with the molecular formula C10H12O. It is a benzaldehyde with an isopropyl group substituted in the 4-position. Cuminaldehyde is a constituent of the essential oils of eucalyptus, myrrh, cassia, cumin, and others. It has a pleasant smell and contributes to the aroma of these oils. It is used commercially in perfumes and other cosmetics. It has been shown that cuminaldehyde, as a small molecule, inhibits the fibrillation of alpha-synuclein, which, if aggregated, forms insoluble fibrils in pathological conditions characterized by Lewy bodies, such as Parkinson's disease, dementia with Lewy bodies and multiple system atrophy. Cuminaldehyde can be prepared synthetically by the reduction of 4-isopropylbenzoyl chloride or by the formylation of cumene. The thiosemicarbazone A thiosemicarbazone is an organosulfur compound with the formula H2NC(S)NHN=CR2. Many variations exist, including those where some or al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionaldehyde
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a pungent and fruity odour. It is produced on a large scale industrially. Production Propionaldehyde is mainly produced industrially by hydroformylation of ethylene: :CO + H2 + C2H4 → CH3CH2CHO In this way, several hundred thousand tons are produced annually. Laboratory preparation Propionaldehyde may also be prepared by oxidizing 1-propanol with a mixture of sulfuric acid and potassium dichromate. The reflux condenser contains water heated at 60 °C, which condenses unreacted propanol, but allows propionaldehyde to pass. The propionaldehyde vapor is immediately condensed into a suitable receiver. In this arrangement, any propionaldehyde formed is immediately removed from the reactor, thus it does not get over-oxidized to propionic acid. Reactions Propionaldehyde exhibits the reactions characteristic of alkyl aldehyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are soluble ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |