|
Close-packed
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a lattice packing is :\frac \approx 0.74048. The same packing density can also be achieved by alternate stackings of the same close-packed planes of spheres, including structures that are aperiodic in the stacking direction. The Kepler conjecture states that this is the highest density that can be achieved by any arrangement of spheres, either regular or irregular. This conjecture was proven by Thomas Hales. The highest density is so far known only for 1, 2, 3, 8, and 24 dimensions. Many crystal structures are based on a close-packing of a single kind of atom, or a close-packing of large ions with smaller ions filling the spaces between them. The cubic and hexagonal arrangements are very cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Close Packing
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or lattice). Carl Friedrich Gauss Johann Carl Friedrich Gauss (; ; ; 30 April 177723 February 1855) was a German mathematician, astronomer, geodesist, and physicist, who contributed to many fields in mathematics and science. He was director of the Göttingen Observatory and ... proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a lattice packing is :\frac \approx 0.74048. The same packing density can also be achieved by alternate stackings of the same close-packed planes of spheres, including structures that are aperiodic in the stacking direction. The Kepler conjecture states that this is the highest density that can be achieved by any arrangement of spheres, either regular or irregular. This conjecture was proven by Thomas Callister Hales, Thomas Hales. The highest d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Packing Factor
In crystallography, atomic packing factor (APF), packing efficiency, or packing fraction is the Packing density, fraction of volume in a crystal structure that is occupied by constituent particles. It is a dimensionless quantity and always less than unity. In atomic systems, by convention, the APF is determined by assuming that atoms are rigid spheres. The radius of the spheres is taken to be the maximum value such that the atoms do not overlap. For one-component crystals (those that contain only one type of particle), the packing fraction is represented mathematically by :\mathrm = \frac where ''N''particle is the number of particles in the unit cell, ''V''particle is the volume of each particle, and ''V''unit cell is the volume occupied by the unit cell. It can be proven mathematically that for one-component structures, the most dense arrangement of atoms has an APF of about 0.74 (see Kepler conjecture), obtained by the close-packing of equal spheres, close-packed structures. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais latices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Crystal System
In crystallography, the hexagonal crystal family is one of the six crystal family, crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section hexagonal crystal family#Crystal systems, crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals. For molecules and polyatomic ions the coordination number of an atom is determined by simply counting the other atoms to which it is bonded (by either single or multiple bonds). For example, [Cr(NH3)2Cl2Br2]− has Cr3+ as its central cation, which has a coordination number of 6 and is described as ''hexacoordinate''. The common coordination numbers are 4, 6 and 8. Molecules, polyatomic ions and coordination complexes In chemistry, coordination number, defined originally in 1893 by Alfred Werner, is the total number of neighbors of a central atom in a molecule or ion. The concept is most commonly applied to coordination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Friedrich Gauss
Johann Carl Friedrich Gauss (; ; ; 30 April 177723 February 1855) was a German mathematician, astronomer, geodesist, and physicist, who contributed to many fields in mathematics and science. He was director of the Göttingen Observatory and professor of astronomy from 1807 until his death in 1855. While studying at the University of Göttingen, he propounded several mathematical theorems. As an independent scholar, he wrote the masterpieces '' Disquisitiones Arithmeticae'' and ''Theoria motus corporum coelestium''. Gauss produced the second and third complete proofs of the fundamental theorem of algebra. In number theory, he made numerous contributions, such as the composition law, the law of quadratic reciprocity and the Fermat polygonal number theorem. He also contributed to the theory of binary and ternary quadratic forms, the construction of the heptadecagon, and the theory of hypergeometric series. Due to Gauss' extensive and fundamental contributions to science ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Closest Packing (CCP) And Hexagonal Closet Packing (HCP)
Cubic may refer to: Science and mathematics * Cube (algebra), "cubic" measurement * Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex ** Cubic crystal system, a crystal system where the unit cell is in the shape of a cube * Cubic function, a polynomial function of degree three * Cubic equation, a polynomial equation (reducible to ''ax''3 + ''bx''2 + ''cx'' + ''d'' = 0) * Cubic form, a homogeneous polynomial of degree 3 * Cubic graph (mathematics - graph theory), a graph where all vertices have degree 3 * Cubic plane curve (mathematics), a plane algebraic curve ''C'' defined by a cubic equation * Cubic reciprocity (mathematics - number theory), a theorem analogous to quadratic reciprocity * Cubic surface, an algebraic surface in three-dimensional space * Cubic zirconia, in geology, a mineral that is widely synthesized for use as a diamond simulacra * CUBIC, a histology method Computing * Cubic IDE, a modular dev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
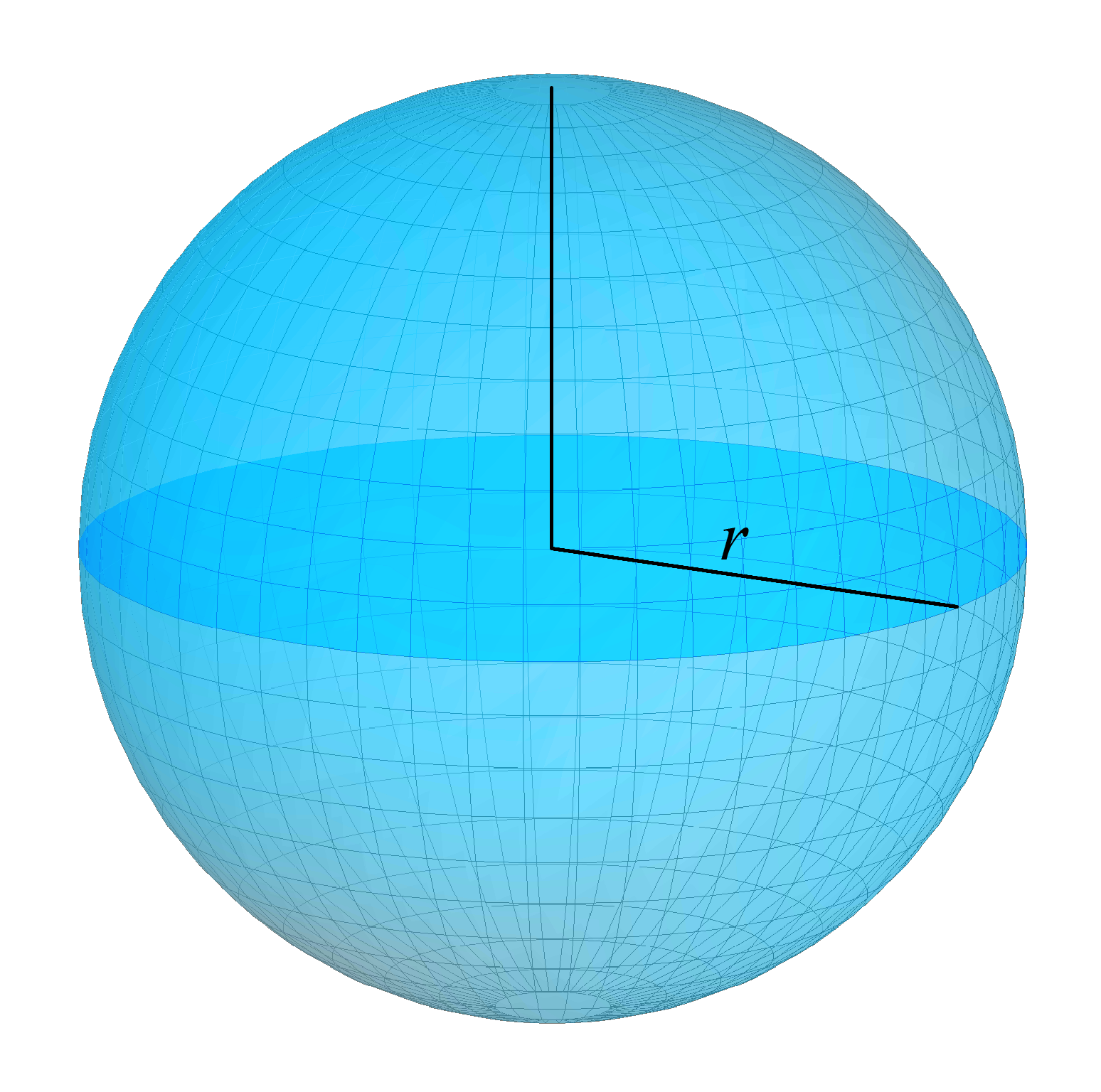
Sphere
A sphere (from Ancient Greek, Greek , ) is a surface (mathematics), surface analogous to the circle, a curve. In solid geometry, a sphere is the Locus (mathematics), set of points that are all at the same distance from a given point in three-dimensional space.. That given point is the center (geometry), ''center'' of the sphere, and the distance is the sphere's ''radius''. The earliest known mentions of spheres appear in the work of the Greek mathematics, ancient Greek mathematicians. The sphere is a fundamental surface in many fields of mathematics. Spheres and nearly-spherical shapes also appear in nature and industry. Bubble (physics), Bubbles such as soap bubbles take a spherical shape in equilibrium. The Earth is spherical Earth, often approximated as a sphere in geography, and the celestial sphere is an important concept in astronomy. Manufactured items including pressure vessels and most curved mirrors and lenses are based on spheres. Spheres rolling, roll smoothly in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Barlow (geologist)
William Barlow Royal Society, FRS (8 August 1845 – 28 February 1934) was an English amateur geologist specialising in crystallography. He was born in Islington, in London, England. His father became wealthy as a speculative builder as well as a building surveyor, allowing William to have a private education. After his father died in 1875, William and his brother inherited this fortune, allowing him to pursue his interest in crystallography without the need to labour for a living. William examined the forms of crystalline structures and deduced that there were only 230 forms of symmetrical crystal arrangements, known as space groups. His results were published in 1894, after they had been independently announced by Evgraf Fedorov and Arthur Schönflies, although his approach did display some novelty. His structural models of simple compounds such as Sodium chloride, NaCl and Caesium chloride, CsCl were later confirmed using X-ray crystallography. He served as the president ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedron
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of irregular octahedra also exist, including both convex set, convex and non-convex shapes. Combinatorially equivalent to the regular octahedron The following polyhedra are combinatorially equivalent to the regular octahedron. They all have six vertices, eight triangular faces, and twelve edges that correspond one-for-one with the features of it: * Triangular antiprisms: Two faces are equilateral, lie on parallel planes, and have a common axis of symmetry. The other six triangles are isosceles. The regular octahedron is a special case in which the six lateral triangles are also equilateral. * Tetragonal bipyramids, in which at least one of the equatorial quadrilaterals lies on a plane. The regular octahedron is a special case in which all thr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diophantine Equation
''Diophantine'' means pertaining to the ancient Greek mathematician Diophantus. A number of concepts bear this name: *Diophantine approximation In number theory, the study of Diophantine approximation deals with the approximation of real numbers by rational numbers. It is named after Diophantus of Alexandria. The first problem was to know how well a real number can be approximated ... * Diophantine equation * Diophantine quintuple * Diophantine set {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |