|
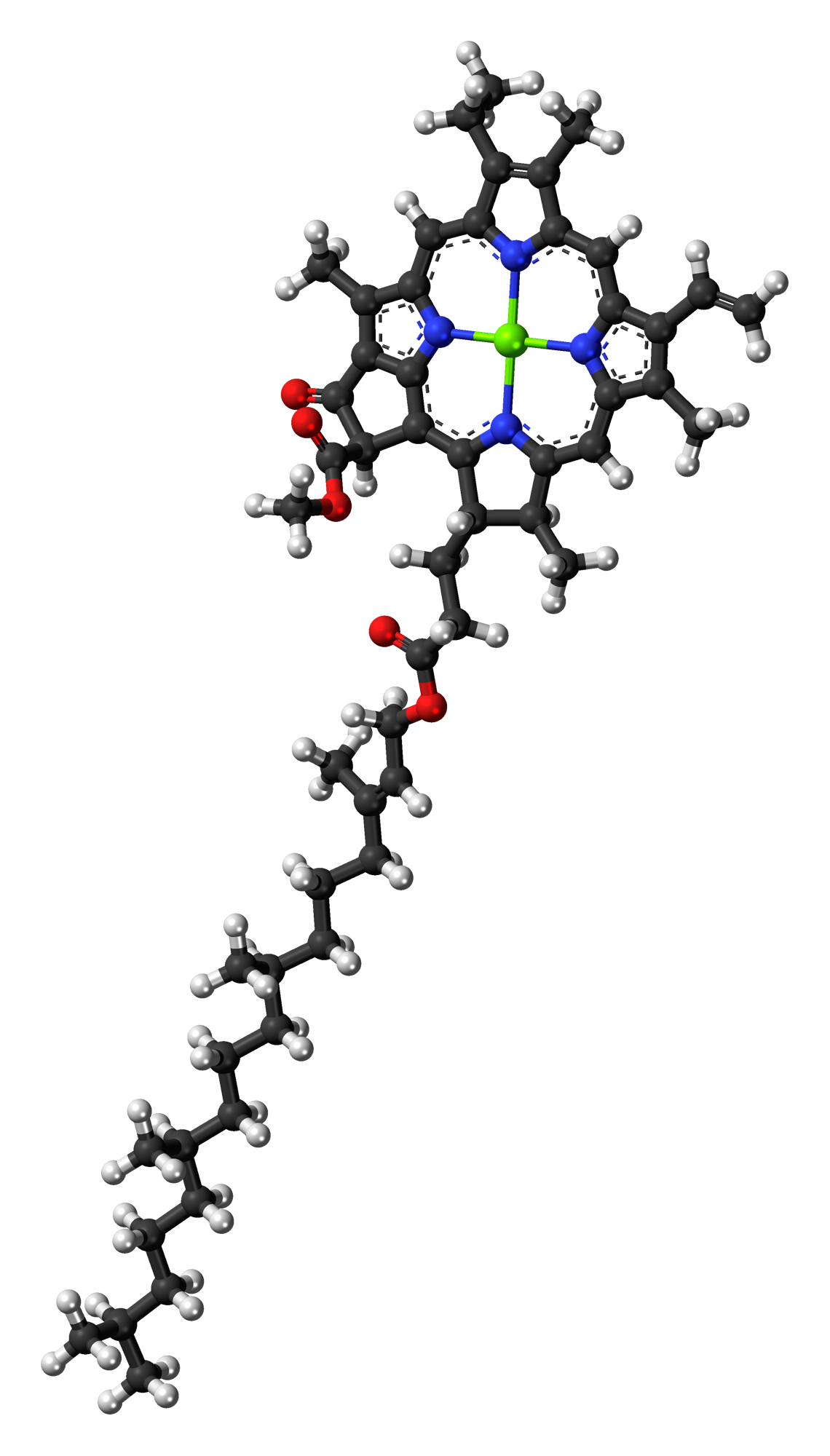
Chlorophyll-a
} Chlorophyll ''a'' is a specific form of chlorophyll used in oxygenic photosynthesis. It absorbs most energy from wavelengths of violet-blue and orange-red light, and it is a poor absorber of green and near-green portions of the spectrum. Chlorophyll does not reflect light but chlorophyll-containing tissues appear green because green light is diffusively reflected by structures like cell walls. This photosynthetic pigment is essential for photosynthesis in eukaryotes, cyanobacteria and prochlorophytes because of its role as primary electron donor in the electron transport chain. Chlorophyll ''a'' also transfers resonance energy in the antenna complex, ending in the reaction center where specific chlorophylls P680 and P700 are located. Distribution of chlorophyll ''a'' Chlorophyll ''a'' is essential for most photosynthetic organisms to release chemical energy but is not the only pigment that can be used for photosynthesis. All oxygenic photosynthetic organisms use chloroph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll
Chlorophyll is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words (, "pale green") and (, "leaf"). Chlorophyll allows plants to absorb energy from light. Those pigments are involved in oxygenic photosynthesis, as opposed to bacteriochlorophylls, related molecules found only in bacteria and involved in anoxygenic photosynthesis. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll ''a'' and ''b''. History Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table), it occurs naturally only in combination with other elements and almost always has an oxidation state of +2. It reacts readily with air to form a thin Passivation (chemistry), passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium Salt (chemistry), salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight magnesium alloy, alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three Helium nucleus, helium nuclei to a carbon nucleus. When such stars explo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P680
P680, or photosystem II primary donor, is the reaction-center chlorophyll ''a'' molecular dimer associated with photosystem II in plants, algae, and cyanobacteria, and central to oxygenic photosynthesis. Etymology Its name is derived from the word “pigment” (P) and the presence of a major bleaching band centered around 680-685 nm in the flash-induced absorbance difference spectra of P680/ P680+•.Shigeru Itoh, S; Iwaki, M; Tomo, T; Satoh, K (1996). Dibromothymoquinone (DBMIB) replaces the function of QA at 77 K in the isolated photosystem II reaction center (Dl-D2-cytochrome 6559) complex: Difference spectrum of the P680+ (DBMIB") state. Plant Cell Physiol. 37(6): 833-839. Components The structure of P680 consists of a hetero dimer of two distinct chlorophyll molecules, referred to as P and P. This “special pair” forms an excitonic dimer that functions as a single unit, excited by light energy as if they were a single molecule. Action and function Excitat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Side Chains
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a larger hydrocarbon backbone. It is one factor in determining a molecule's properties and reactivity. A side chain is also known as a pendant chain, but a pendant group (side group) has a different definition. Conventions The placeholder R is often used as a generic placeholder for alkyl (saturated hydrocarbon) group side chains in structural formulae. To indicate other non-carbon groups in structure diagrams, X, Y, or Z are often used. History The ''R'' symbol was introduced by 19th-century French chemist Charles Frédéric Gerhardt, who advocated its adoption on the grounds that it would be widely recognizable and intelligible given its correspondence in multiple European languages to the initial letter of "root" or "residue": French ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorin
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenation, hydrogenated porphyrins. The parent chlorin is an unstable compound which undergoes air oxidation to porphine. The name chlorin derives from chlorophyll. Chlorophylls are magnesium-containing chlorins and occur as photosynthetic pigments in chloroplasts. The term "chlorin" strictly speaking refers to only compounds with the same ring oxidation state as chlorophyll. Chlorins are excellent photosensitizing agents. Various synthetic chlorins analogues such as Temoporfin, m-tetrahydroxyphenylchlorin (mTHPC) and Talaporfin, mono-L-aspartyl chlorin e6 are effectively employed in experimental photodynamic therapy as photosensitizer. Chlorophylls The most abundant chlorin is the Photosynthesis, photosynthetic pigment chlorophyll. Chlorophylls have a fifth, ketone-containing ring unlike the chlorins. Diverse chlorophylls exists, such as Chlorophyll a, chlorophyll ''a'', Chlorophyll b, chlorophyll ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anoxygenic Photosynthesis
Anoxygenic photosynthesis is a special form of photosynthesis used by some bacteria and archaea, which differs from the better known oxygenic photosynthesis in plants in the reductant used (e.g. hydrogen sulfide instead of water) and the byproduct generated (e.g. elemental sulfur instead of molecular oxygen). Unlike oxygenic phototrophs that only use the Calvin cycle to fix carbon dioxide, anoxygenic phototrophs can use both the Calvin cycle and the reverse TCA cycle to fix carbon dioxide. Additionally, unlike its oxygenic counterpart that predominantly uses chlorophyll, this type of photosynthesis uses the bacteriochlorophyll, BChl to utilize light as an energy source. A precursor to oxygenic photosynthesis but having been developed after chemolithoautotrophy, anoxygenic photosynthesis uses one of two reaction centers while oxygenic photosynthesis uses both type I and type II reaction centers. The rise of anoxygenic photosynthesis During the Archean Era on Earth, the atm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriochlorophyll
Bacteriochlorophylls (BChl) are photosynthetic pigments that occur in various phototrophic bacteria. They were discovered by C. B. van Niel in 1932. They are related to chlorophylls, which are the primary pigments in plants, algae, and cyanobacteria. Organisms that contain bacteriochlorophyll conduct photosynthesis to sustain their energy requirements, but the process is anoxygenic and does not produce oxygen as a byproduct. They use wavelengths of light not absorbed by plants or cyanobacteria. Replacement of with protons gives bacteriophaeophytin (BPh), the phaeophytin form. BacterioChlorophyll a.svg, bacteriochlorophyll ''a'' BacterioChlorophyll b.svg, bacteriochlorophyll ''b'' BacterioChlorophyll c.svg, bacteriochlorophyll ''c'' BacterioChlorophyll d.svg, bacteriochlorophyll ''d'' BacterioChlorophyll e.svg, bacteriochlorophyll ''e'' Bacteriochlorophyll f.svg, bacteriochlorophyll ''f'' BacterioChlorophyll g.svg, bacteriochlorophyll ''g'' Structure Bacteriochlorophyll ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoautotroph
Photoautotrophs are organisms that can utilize light energy from sunlight, and elements (such as carbon) from inorganic compounds, to produce organic materials needed to sustain their own metabolism (i.e. autotrophy). Such biological activities are known as photosynthesis, and examples of such organisms include plants, algae and cyanobacteria. Eukaryotic photoautotrophs absorb photonic energy through the photopigment chlorophyll (a porphyrin derivative) in their endosymbiont chloroplasts, while prokaryotic photoautotrophs use chlorophylls and bacteriochlorophylls present in free-floating cytoplasmic thylakoids. Plants, algae, and cyanobacteria perform oxygenic photosynthesis that produces oxygen as a byproduct, while some bacteria perform anoxygenic photosynthesis. Origin and the Great Oxidation Event Chemical and geological evidence indicate that photosynthetic cyanobacteria existed about 2.6 billion years ago and anoxygenic photosynthesis had been taking place since a b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anaerobic Organism
An anaerobic organism or anaerobe is any organism that does not require oxygen, molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenated environment. Anaerobes may be unicellular (e.g. protozoans, bacteria) or multicellular. Most fungi are obligate aerobes, requiring oxygen to survive. However, some species, such as the Chytridiomycota that reside in the rumen of cattle, are obligate anaerobes; for these species, anaerobic respiration is used because oxygen will disrupt their metabolism or kill them. The sea floor is possibly one of the largest accumulation of anaerobic organisms on Earth, where microbes are primarily concentrated around Hydrothermal_vent, hydrothermal vents. These microbes produce energy in absence of sunlight or oxygen through a process called chemosynthesis, whereby inorganic compounds such as hydrogen gas, hydrogen sulfide or ferrous ions are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green Sulfur Bacteria
The green sulfur bacteria are a phylum, Chlorobiota, of obligately anaerobic photoautotrophic bacteria that metabolize sulfur. Green sulfur bacteria are nonmotile (except ''Chloroherpeton thalassium'', which may glide) and capable of anoxygenic photosynthesis. They live in anaerobic aquatic environments. In contrast to plants, green sulfur bacteria mainly use sulfide ions as electron donors. They are autotrophs that utilize the reverse tricarboxylic acid cycle to perform carbon fixation. They are also mixotrophs and reduce nitrogen. Characteristics Green sulfur bacteria are gram-negative rod or spherical shaped bacteria. Some types of green sulfur bacteria have gas vacuoles that allow for movement. They are photolithoautotrophs, and use light energy and reduced sulfur compounds as the electron source. Electron donors include , , S. The major photosynthetic pigment in these bacteria is Bacteriochlorophylls ''c'' or ''d'' in green species and ''e'' in brown species, and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll B
} Chlorophyll ''b'' is a form of chlorophyll. Chlorophyll ''b'' helps in photosynthesis by absorbing light energy. It is more soluble than chlorophyll ''a'' in polar solvents because of its carbonyl group. Its color is green, and it primarily absorbs blue light. In land plants, the light-harvesting antennae around photosystem II contain the majority of chlorophyll ''b''. Hence, in shade-adapted chloroplasts, which have an increased ratio of photosystem II to photosystem I, there is a higher ratio of chlorophyll ''b'' to chlorophyll ''a''. This is adaptive, as increasing chlorophyll ''b'' increases the range of wavelengths absorbed by the shade chloroplasts. Biosynthesis The Chlorophyll ''b'' biosynthetic pathway utilizes a variety of enzymes. In most plants, chlorophyll is derived from glutamate and is synthesised along a branched pathway that is shared with heme and siroheme. The initial steps incorporate glutamic acid into 5-aminolevulinic acid (ALA); two molecules o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |