|
Chemical Pulping
Paper chemicals designate a group of chemicals that are used for paper manufacturing, or modify the properties of paper. These chemicals can be used to alter the paper in many ways, including changing its color and brightness, or by increasing its strength and resistance to water. The chemicals can be defined on basis of their usage in the process. Chemical usage is not only for imparting properties to paper but to handle the water cycles in the process, conditioning of fabrics, cleaning of equipment and several other applications. Chemicals used in paper manufacturing Pulping Chemical pulping involves dissolving lignin in order to extract the cellulose from the wood fiber. The different processes of chemical pulping include the Kraft process, which uses caustic soda and sodium sulfide and is the most common; alternatively, the use of sulfurous acid is known as the sulfite process, the neutral sulfite semichemical is treated as a third process separate from sulfite, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Substance
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combined without reacting, they may form a chemical mixture. If a mixture is separated to isolate one chemical substance to a desired degree, the resulting substance is said to be chemically pure. Chemical substances can exist in several different physical states or phases (e.g. solids, liquids, gases, or plasma) without changing their chemical composition. Substances transition between these phases of matter in response to changes in temperature or pressure. Some chemical substances can be combined or converted into new substances by means of chemical reactions. Chemicals that do not possess this ability are said to be inert. Pure water is an example of a chemical substance, with a constant composition of two hydrogen atoms bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guar Gum
Guar gum, also called guaran, is a galactomannan polysaccharide extracted from guar beans that has thickening and stabilizing properties useful in food, feed, and industrial applications. The guar seeds are mechanically dehusked, hydrated, milled and screened according to application. It is typically produced as a free-flowing, off-white powder. Production and trade The guar bean is principally grown in India, Pakistan, the United States, Australia and Africa. India is the largest producer, accounting for nearly 80% of world production. In India, Rajasthan, Gujarat, and Haryana are the main producing regions. The US has produced 4,600 to 14,000 tonnes of guar over the last 5 years. Texas acreage since 1999 has fluctuated from about 7,000 to 50,000 acres. The world production for guar gum and its derivatives is about 1.0 million tonnes. Non-food guar gum accounts for about 40% of the total demand. Properties Chemical composition Chemically, guar gum is an exo- polysac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
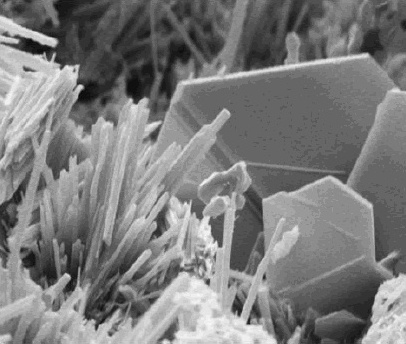
Rosin
Rosin (), also known as colophony or Greek pitch (), is a resinous material obtained from pine trees and other plants, mostly conifers. The primary components of rosin are diterpenoids, i.e., C20 carboxylic acids. Rosin consists mainly of resin acids, especially abietic acid. Rosin often appears as a semi-transparent, brittle substance that ranges in color from yellow to black and melts at stove-top temperatures. In addition to industrial applications such as in varnishes, adhesives, and sealing wax, rosin is used with string instruments on the bow hair to enhance its ability to grip and sound the strings, and it provides grip in various sports and activities. Rosin also serves as an ingredient in medicinal and pharmaceutical formulations and can cause contact dermatitis or occupational asthma in sensitive individuals. It is an FDA approved food additive. The name "colophony" originates from , Latin for "resin from Colophon" (), an ancient Ionic city. Properties R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 chemical structure, structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetism, diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Hydroxide
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk of magnesia. Preparation Treating the solution of different soluble magnesium salts with alkaline water induces the Precipitation (chemistry), precipitation of the solid hydroxide Mg(OH)2: :Mg2+ + 2 OH− → Mg(OH)2 As is the second most abundant cation present in seawater after , it can be economically extracted directly from seawater by alkalinisation as described here above. On an industrial scale, Mg(OH)2 is produced by treating seawater with calcium hydroxide, lime (Ca(OH)2). A volume of of seawater gives about of Mg(OH)2. Ca(OH)2 ) is far more soluble than Mg(OH)2 ) and dramatically increases the pH value of seawater from 8.2 to 12.5. The less soluble precipitates because of the common ion effect due to the added by the di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide. Calcium hydroxide has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Solubility Calcium hydroxide is moderately soluble in water, as seen for many dihydroxides. Its solubility increases from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. Its solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Carbonate
Magnesium carbonate, (archaic name magnesia alba), is an inorganic salt that is a colourless or white solid. Several hydrated and Base (chemistry), basic forms of magnesium carbonate also exist as minerals. Forms The most common magnesium carbonate forms are the anhydrous salt called magnesite (), and the di, tri, and pentahydrates known as barringtonite (), nesquehonite (), and lansfordite (), respectively. Some basic forms such as artinite (), hydromagnesite (), and dypingite () also occur as minerals. All of those minerals are colourless or white. Magnesite consists of colourless or white trigonal crystals. The anhydrous salt is practically insoluble in water, acetone, and ammonia. All forms of magnesium carbonate react with acids. Magnesite crystallizes in the calcite structure wherein magnesium, is Coordination geometry#Crystallography usage, surrounded by six oxygen atoms. The dihydrate has a triclinic structure, while the trihydrate has a monoclinic structure. Refe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Bisulfite
Magnesium is a chemical element; it has symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table), it occurs naturally only in combination with other elements and almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single compound calcium oxide. Calcium oxide that survives processing without reacting in building products, such as cement, is called free lime. Quicklime is relatively inexpensive. Both it and the chemical derivative calcium hydroxide (of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypochlorous Acid
Hypochlorous acid is an inorganic compound with the chemical formula , also written as HClO, HOCl, or ClHO. Its structure is . It is an acid that forms when chlorine dissolves in water, and itself partially dissociates, forming a hypochlorite anion, . HClO and are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine. Because of its strong antimicrobial properties, the related compounds sodium hypochlorite (NaOCl) and calcium hypochlorite () are ingredients in many commercial bleaches, deodorants, and disinfectants. The white blood cells of mammals, such as humans, also contain hypochlorous acid as a tool against foreign bodies. In living organisms, HOCl is generated by the reaction of hydrogen peroxide with chloride ions under the catalysis of the heme enzyme myeloperoxidase (MPO). Like many other disinfectants, hypochlorous acid solutions will destroy pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |