|
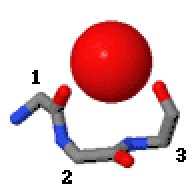
Catgrip
Catgrips are small cation-binding molecular features of proteins and peptides. Each consists of the main chain atoms only of three consecutive amino acid residues. The first and third main chain CO groups bind the cations, often calcium, magnesium, potassium or sodium, with no side chain involvement. Many catgrips bind a water molecule instead of a cation; it is hydrogen-bonded to the first and third main chain CO groups. Catgrips are found as calcium-binding features in annexins, matrix metalloproteinases (e.g.serralysins), subtilisins and phospholipase A2. They are also observed in synthetic peptides and in cyclic hexapeptides made from alternating D,L amino acids. The conformation of a catgrip is such that the CO groups of the first and third amino acid residues are liable to be electrostatically attracted or hydrogen bonded to a positively charged, or partially positively charged, atom or group. Catgrips are defined by the phi, psi main chain dihedral angles of the second and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Niche (protein Structural Motif)
In the area of protein structural motifs, niches are three or four amino acid residue features in which main-chain CO groups are bridged by positively charged or δ+ groups. The δ+ groups include groups with two hydrogen bond donor atoms such as NH2 groups and water molecules. In typical proteins, 7% of amino acid residues belong to niches bound to a δ+ group, while another 7% have the conformation but no single cationic bridging group is detected. Niches are of two kinds, distinguished as niche3 (3 residues, ''i'' to ''i+2'') and niche4 (4 residues, ''i'' to ''i+3''). In a niche3 motif the δ+-binding carbonyl groups are from residues ''i'' and ''i+2'' while in a niche4 motif they are from residues ''i'' and ''i+3''. A niche3 has the α conformation for residue ''i+1'' and the β conformation for residue ''i+2''; a niche4 has the α conformation for residues ''i+1'' and ''i+2'' and the β conformation for residue ''i+3''. A niche occurs commonly at the C-terminus of α-helices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component ( water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide ( lye) is used in soap manufacture, and sodium chloride ( edible salt) is a de-icing agent and a nutrient for animals i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annexin
Annexin is a common name for a group of cellular proteins. They are mostly found in eukaryotic organisms (animal, plant and fungi). In humans, the annexins are found inside the cell (biology), cell. However some annexins (Annexin A1, Annexin A2, and Annexin A5) can be Secretion, secreted from the cytoplasm to outside cellular environments, such as blood. Annexin is also known as ''lipocortin''. Lipocortins suppress phospholipase A2. Increased expression of the gene coding for annexin-1 is one of the mechanisms by which glucocorticoids (such as cortisol) inhibit inflammation. Introduction The protein family of annexins has continued to grow since their association with intracellular membranes was first reported in 1977. The recognition that these proteins were members of a broad family first came from protein sequence comparisons and their cross-reactivity with antibodies. One of these workers (Geisow) coined the name Annexin shortly after. As of 2002 160 annexin proteins hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix Metalloproteinase
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily. Collectively, these enzymes are capable of degrading all kinds of extracellular matrix proteins, but also can process a number of Biological activity, bioactive molecules. They are known to be involved in the cleavage of cell surface Receptor (biochemistry), receptors, the release of apoptosis, apoptotic ligands (such as the FAS ligand), and chemokine/cytokine inactivation. MMPs are also thought to play a major role in cell behaviors such as cell proliferation, cell migration, migration (cell adhesion, adhesion/dispersion), Cellular differentiation, differentiation, angiogenesis, apoptosis, and Immune system, host defense. They were first described in vertebrates (19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serralysin
Serralysin (, ''Pseudomonas aeruginosa alkaline proteinase'', ''Escherichia freundii proteinase'', ''Serratia marcescens extracellular proteinase'', ''Serratia marcescens metalloproteinase'', ''Pseudomonas aeruginosa alk. protease'', ''Serratia marcescens metalloprotease'') is an enzyme. This enzyme catalyses the following chemical reaction : Preferential cleavage of bonds with hydrophobic residues in P1' This extracellular endopeptidase is present in ''Pseudomonas aeruginosa'', '' Escherichia freundii'', ''Serratia marcescens'' and ''Erwinia chrysanthemi ''Dickeya dadantii'' is a gram-negative bacillus that belongs to the family Pectobacteriaceae. It was formerly known as ''Erwinia chrysanthemi'' but was reassigned as ''Dickeya dadantii'' in 2005. Members of this family are facultative anaerobes ...''. References External links * {{Portal bar, Biology, border=no EC 3.4.24 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subtilisin
Subtilisin is a protease (a protein-digesting enzyme) initially obtained from ''Bacillus subtilis''. Subtilisins belong to subtilases, a group of serine proteases that – like all serine proteases – initiate the nucleophilic attack on the peptide (amide) bond through a serine residue at the active site. Subtilisins typically have molecular weights 27kDa. They can be obtained from certain types of soil bacteria, for example, '' Bacillus amyloliquefaciens'' from which they are secreted in large amounts. Nomenclature Subtilisin is also commercially known as ''Alcalase®'', ''Endocut-02L'', ''ALK-enzyme'', ''bacillopeptidase'', ''Bacillus subtilis alkaline proteinase bioprase'', ''bioprase AL'', ''colistinase'', ''genenase I'', ''Esperase®'', ''maxatase'', ''protease XXVII'', ''thermoase'', ''superase'', ''subtilisin DY'', ''subtilopeptidase'', ''SP 266'', ''Savinase®'', ''kazusase'', ''protease VIII'', ''protin A 3L'', ''Savinase®'', ''orientase 10B'', ''protease S. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |