|
Carbon–hydrogen Bond Activation
In organic chemistry and organometallic chemistry, carbon–hydrogen bond activation ( activation) is a type of organic reaction in which a carbon–hydrogen bond is Bond cleavage, cleaved and replaced with a bond (X ≠ H is typically a main group element, like carbon, oxygen, or nitrogen). Some authors further restrict the term C–H activation to reactions in which a C–H bond, one that is typically considered to be "unreactive", interacts with a transition metal center M, resulting in its cleavage and the generation of an organometallic species with an M–C bond. The organometallic intermediate resulting from this step (sometimes known as the activation step) could then undergo subsequent reactions with other reagents, either ''in situ'' (often allowing the transition metal to be used in a Catalysis, catalytic amount) or in a separate step, to produce the Late-stage functionalization, functionalized product. The alternative term functionalization is used to describe any ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene radical, methylene, the parent hydride from which all other carbene compounds are formally derived. There are two types of carbenes: singlet state, singlets or triplet state, triplets, depending upon their electronic structure. The different classes undergo different reactions. Most carbenes are extremely reactive and short-lived. A small number (the diHalogen, halocarbenes, carbon monoxide, and carbon monosulfide) can be isolated, and can stabilize as Coordination complex, metal ligands, but otherwise cannot be stored in bulk. A rare exception are the persistent carbenes, which have extensive application in modern organometallic chemistry. Generatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomercury
Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Many organomercury compounds are highly toxic, but some are used in medicine, e.g., merbromin ("Mercurochrome") and the vaccine preservative thiomersal. Structure and bonding Most organomercury compounds feature diamagnetic Hg(II) and adopt a linear C-Hg-X structure. Indeed, no organic derivatives of Hg are known, as Hg requires electronegative subtituents for condensed-phase stability. Hg(II) derivatives are neither Lewis basic or Lewis acidic. They are stable to oxygen and water, indicating the low polarity of the Hg-C bond. Mercury forms a compound with two cyclopentadiene ligands, but the resulting complex may not be a metallocene. When made in the 1950s, it was too sensitive for structural determination. Toxicity The toxicity of organomercury compounds presents both dangers and benefits. Dimethylmercury in particular is notoriously toxic, but found use as an antifungal a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Acetate
Mercury(II) acetate, also known as mercuric acetate is a chemical compound, the mercury(II) salt of acetic acid, with the formula Hg( O2 CC H3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white, water-soluble solid, but some samples can appear yellowish with time owing to decomposition. Structure Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å. Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg. Synthesis and reactions Mercury(II) acetate can be produced by reaction of mercuric oxide with acetic acid. HgO + 2 CH3COOH → Hg(CH3COO)2 + H2O Inorganic reactions Mercury(II) acetate in acetic acid solution reacts with H2S to rapidly precipitate the black (β) polymorph of HgS. With ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Otto Dimroth
Otto Dimroth (28 March 1872 – 16 May 1940) was a German chemist A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of .... He is known for the Dimroth rearrangement, as well as a type of condenser with an internal double spiral, the Dimroth condenser. He made pioneering contributions to organomercury chemistry. His son Karl Dimroth was also a renowned chemist, who described the first synthesis of 3-benzoxepin. References * Academic staff of the University of Greifswald 1872 births 1940 deaths {{germany-scientist-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked with the double dagger (‡) symbol. As an example, the transition state shown below occurs during the SN2 reaction of bromoethane with a hydroxide anion: The activated complex of a reaction can refer to either the transition state or to other states along the reaction coordinate between reactants and products, especially those close to the transition state. Peter Atkins and Julio de Paula, ''Physical Chemistry'' (8th ed., W.H. Freeman 2006), p.809 According to the transition state theory, once the reactants have passed through the transition state configuration, they always continue to form products. History of concept The concept of a transition state has been important in many theories of the rates at which chemical re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-bond Metathesis
In organometallic chemistry, sigma-bond metathesis is a chemical reaction wherein a metal-ligand sigma bond undergoes metathesis (exchange of parts) with the sigma bond in some reagent. The reaction is illustrated by the exchange of lutetium(III) methyl complex with a hydrocarbon (R-H): :(C5Me5)2Lu-CH3 + R-H → (C5Me5)2Lu-R + CH4 This reactivity was first observed by Patricia Watson, a researcher at duPont.{{cite journal, author=Watson, Patricia, title=Methane exchange reactions of lanthanide and early-transition-metal methyl complexes, journal=Journal of the American Chemical Society, year=1983, volume=32, pages=6491–6493, doi=10.1021/ja00359a023 The reaction is mainly observed for complexes of metals with d0 configuration, e.g. complexes of Sc(III), Zr(IV), Nb(V), Ta(V), etc. f-Element complexes also participate, regardless of the number of f-electrons. The reaction is thought to proceed via cycloaddition. Indeed, the rate of the reaction is characterized by a highly n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concerted Metalation Deprotonation
Concerted metalation-deprotonation (CMD) is a mechanistic pathway through which transition-metal catalyzed Carbon–hydrogen bond activation, C–H activation reactions can take place. In a CMD pathway, the C–H bond of the substrate is cleaved and the new C–Metal bond forms through a single transition state. This process does not go through a metal hydride species that is bound to the cleaved hydrogen atom. Instead, a carboxylate or carbonate base Deprotonation, deprotonates the substrate. The first proposal of a concerted metalation deprotonation pathway was by S. Winstein and T. G. Traylor in 1955 for the acetolysis of diphenylmercury. It was found to be the lowest energy transition state in a number of computational studies, was experimentally confirmed through Nuclear magnetic resonance, NMR experiments, and has been hypothesized to occur in mechanistic studies. While there are a number of different possible mechanisms for C–H activation, a CMD pathway is common for high va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
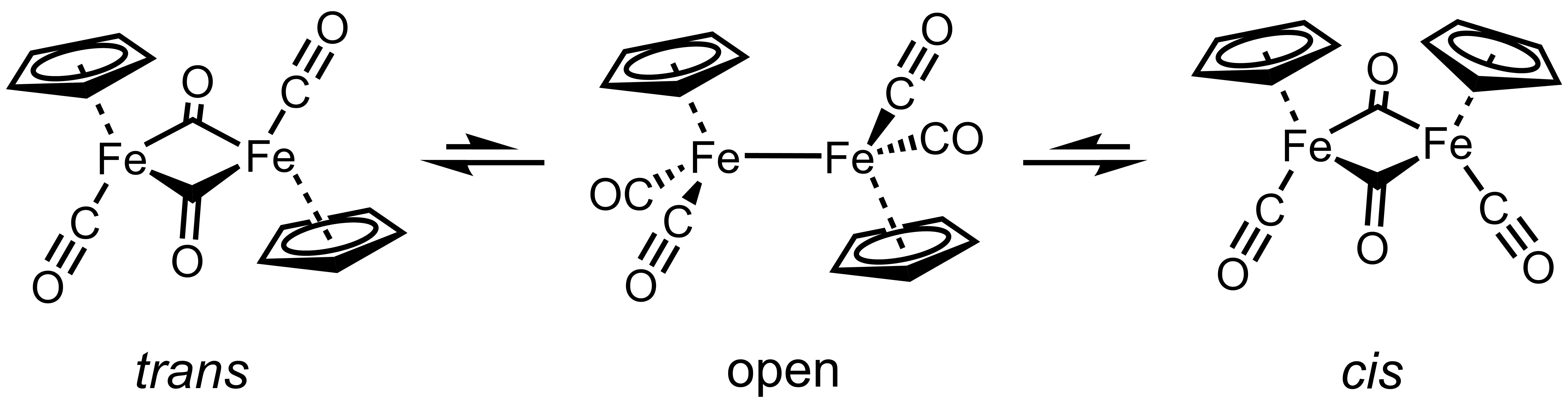
Cyclopentadienyliron Dicarbonyl Dimer
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula ''η''5-C5H5)Fe(CO)2sub>2, often abbreviated to Cp2Fe2(CO)4, pFe(CO)2sub>2 or even Fp2, with the colloquial name "fip dimer". It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water. Cp2Fe2(CO)4 is reasonably stable to storage under air and serves as a convenient starting material for accessing other Fp (CpFe(CO)2) derivatives. Structure In solution, Cp2Fe2(CO)4 can be considered a dimeric half-sandwich complex. It exists in three isomeric forms: ''cis'', ''trans'', and an unbridged, open form. These isomers are distinguished by the position of the ligands. The ''cis'' and ''trans'' isomers differ in the relative position of C5H5 (Cp) ligands. The ''cis'' and ''trans'' isomers have the formulatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |