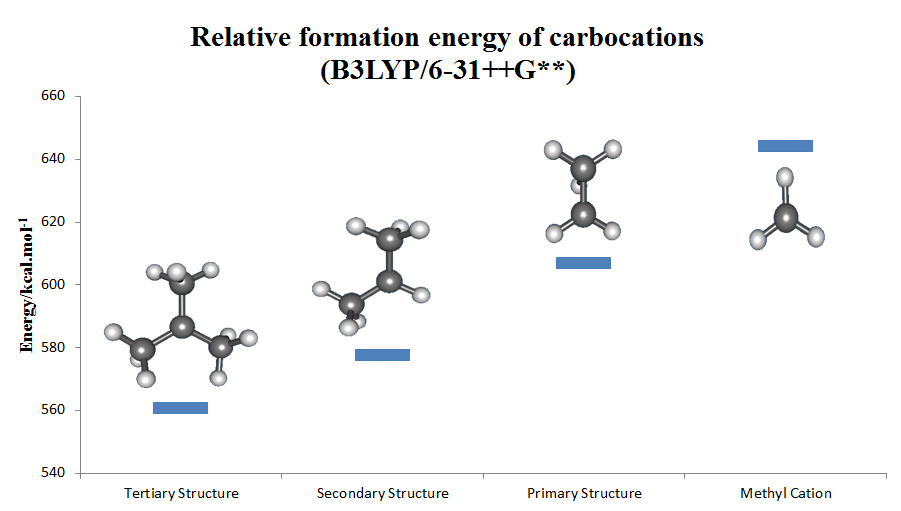
|
Carbocationic
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. Among the simplest carbocations are the methenium (a carbenium ion), methanium (a carbonium ion), acylium ions , and vinyl cations. Until the early 1970s, carbocations were called ''carbonium ions''. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. However, others have more narrowly defined the term 'carbonium ion' as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbenium Ion
The carbenium ion is a kind of cation, positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a general term for diamagnetic carbon-based cations. In parallel with carbenium ions is another subset of carbocations, the carbonium ions with the formula R5+. In carbenium ions charge is localized. They are isoelectronic with monoboranes such as B(CH3)3. Nomenclature Reactivity Carbenium ions are generally highly reactive due to having an incomplete octet rule, octet of electrons; however, certain carbenium ions, such as the tropylium cation, tropylium ion, are relatively stable due to the positive charge being delocalised between the carbon atoms.(It can even exist stably in aqueous solution.) Rearrangements Carbenium ions sometimes rearrangement reaction, rearrange readily. For example, when pentan-3-ol is heated with aq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-butyl Cation Resonance (cropped)
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanium
In chemistry, methanium is a complex positive ion with formula (metastable transitional form, a carbon atom covalently bonded to five hydrogen atoms) or (fluxional form, namely a molecule with one carbon atom covalently bonded to three hydrogen atoms and one dihydrogen molecule), bearing a +1 electric charge. It is a superacid and one of the onium ions, indeed the simplest carbonium ion. It is highly unstable and highly reactive even upon having a complete octet, thus granting its superacidic properties. Methanium can be produced in the laboratory as a rarefied gas or as a dilute species in superacids. It was prepared for the first time in 1950 and published in 1952 by Victor Talrose and his assistant Anna Konstantinovna Lyubimova. It occurs as an intermediate species in chemical reactions. The methanium ion is named after methane (), by analogy with the derivation of ammonium ion () from ammonia (). Structure Fluxional methanium can be visualised as a carbenium ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperconjugation
In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electrons in a sigma (σ) orbital (e.g. C–H or C–C) with an adjacent unpopulated non-bonding p or antibonding σ* or π* orbitals to give a pair of extended molecular orbitals. However, sometimes, low-lying antibonding σ* orbitals may also interact with filled orbitals of lone pair character (n) in what is termed '' negative hyperconjugation''. Increased electron delocalization associated with hyperconjugation increases the stability of the system. In particular, the new orbital with bonding character is stabilized, resulting in an overall stabilization of the molecule. Only electrons in bonds that are in the β position can have this sort of direct stabilizing effect — donating from a sigma bond on an atom to an orbital in another ato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontier orbitals'', such as in the frontier molecular orbital theory. Gap The energy difference between the HOMO and LUMO is ''the HOMO–LUMO gap''. Its size can be used to predict the strength and stability of transition metal Coordination complex, complexes, as well as the colors they produce in solution. As a rule of thumb, the smaller a compound's HOMO–LUMO gap, the less stable the compound. Recent quantum‐chemical analyses of over 700 compounds demonstrated that terrestrial secondary metabolites exhibit HOMO–LUMO gaps on average about 2 eV narrower than organic molecules found in carbonaceous meteorites, and that combining gap width with hydrophilicity creates a robust discriminator between biotic and abiotic chemistries. This sugges ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a Tetrahedral molecular geometry, tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed ou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bent's Rule
In chemistry, Bent's rule describes and explains the relationship between the orbital hybridization and the electronegativities of substituents. The rule was stated by Henry A. Bent as follows: Valence bond theory gives a good approximation of molecular structure. Bent's rule addresses disparities between the observed and idealized geometries. According to Bent's rule, a central atom bonded to multiple groups will rehybridize so that orbitals with more s character are directed towards electropositive groups, and orbitals with more p character will be directed towards groups that are more electronegative. By removing the assumption that all hybrid orbitals are equivalent, Bent's rule leads to improved predictions of molecular geometry and bond strengths. Bent's rule can be justified through the relative energy levels of ''s'' and ''p'' orbitals. Bent's rule represents a modification of VSEPR theory for molecules of lower than ideal symmetry. For bonds with the larger atoms fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VSEPR Theory
Valence shell electron pair repulsion (VSEPR) theory ( , ) is a conceptual model, model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Sydney Nyholm, Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other. The greater the repulsion, the higher in energy (less stable) the molecule is. Therefore, the VSEPR-predicted molecular geometry of a molecule is the one that has as little of this repulsion as possible. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) metho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and unlike charges attract each other. An object with no net charge is referred to as neutral particle, electrically neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum mechanics, quantum effects. In an isolated system, the total charge stays the same - the amount of positive charge minus the amount of negative charge does not change over time. Electric charge is carried by subatomic particles. In ordinary matter, negative charge is carried by electrons, and positive charge is carried by the protons in the atomic nucleus, nuclei of atoms. If there are more electrons than protons in a piece of matter, it will have a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octet Rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens; although more generally the rule is applicable for the s-block and p-block of the periodic table. Other rules exist for other elements, such as the duplet rule for hydrogen and helium, and the 18-electron rule for transition metals. The valence electrons in molecules like carbon dioxide (CO₂) can be visualized using a Lewis electron dot diagram. In covalent bonds, electrons shared between two atoms are counted toward the octet of both atoms. In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in both the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence Electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical properties, such as its valence—whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration. For a main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner shell. An atom with a closed shell of valence electrons (corresponding to a noble gas configuration) tends to be chemically inert. Atoms with one or two valence electrons more than a closed shell are highly reactive due to the relativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical (or hypothetical) contributing structures. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is suffi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |