|
Benzeneazophenol
Solvent Yellow 7 is an aromatic organic molecule and a common azo dye with a formula of C6H5N2C6H4OH. It has a phenolic hydroxyl and an azo group in the same molecule. Synthesis Like most azobenzenes, Solvent Yellow 7 can be synthesized by the reaction of the phenyldiazonium salt with phenol. The optimal pH value for this azo coupling is 8.5-10. The reaction is carried out in water, since sodium chloride (or potassium chloride) formed in the reaction is soluble in water, while the product precipitates. As azo dyes are not usually water soluble, the effect of various solvents on them has been studied analytically, and likewise analytical methods and calculations for the color concentration developed. Further reactions The molecule can be further reacted including with bromine, and other halogens. Other reactions include nitration. The reactivity with Grignard reagents has also been studied. Toxicology The toxicology has been extensively studied, including IARC studies. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived , referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a free element in nature. Instead, it can be isolated from colourless soluble crystalline mineral halide Ionic salt, salts analogous to table salt, a property it shares with the other halogens. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its Bromine cycle, accumulation in the oceans. Commercially the element is easily extracted from brine evaporation ponds, mostly in the United States and Israel. The mass of bromine in the oce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
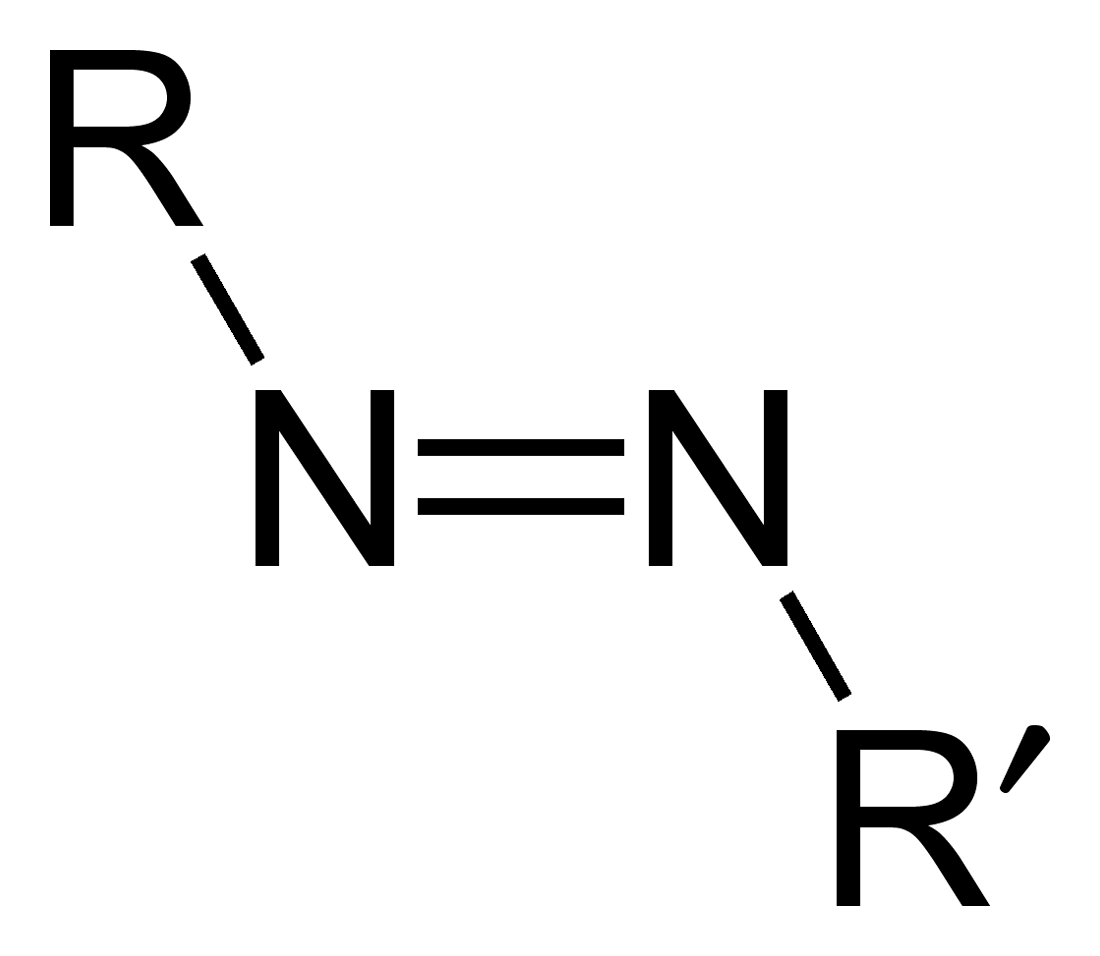
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene.", where Ph stands for phenyl group. The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds urinary tract infections">Phenazopyridine, an aryl azo compound, is used to treat urinary tract infections">150px Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent Yellow 1
Aniline Yellow is a yellow azo dye and an aromatic amine. It is a derivative of azobenzene. It has the appearance of an orange powder. Aniline Yellow was the first azo dye. it was first produced in 1861 by C. Mene. The second azo dye was Bismarck Brown in 1863. Aniline Yellow was commercialized in 1864 as the first commercial azo dye, a year after aniline black. It is manufactured from aniline. Uses Aniline Yellow is used in microscopy for vital staining, in pyrotechnics for yellow colored smokes, in yellow pigments and inks including inks for inkjet printers. It is also used in insecticides, lacquers, varnishes, waxes, oil stains, and styrene resins. It is also an intermediate in synthesis of other dyes, e.g. chrysoidine, indulines, Solid Yellow, and Acid Yellow. Safety Aminoazobenzene compounds are often carcinogenic. See also *Direct Yellow 4 *Titan yellow Titan yellow is a compound with formula C28H19N5Na2O6S4. It is a triazene dye used as a stain and fluorescent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene.", where Ph stands for phenyl group. The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds urinary tract infections">Phenazopyridine, an aryl azo compound, is used to treat urinary tract infections">150px Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon–carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. Grignard reagents are rarely isolated as solids. Instead, they are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran using air-free techniques. Grignard reagents are complex with the magnesium atom bonded to two ether ligands as well as the halide and organyl ligands. The discovery of the Grignard reaction in 1900 was recogn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group () into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters () between Alcohol (chemistry), alcohols and nitric acid (as occurs in the Organic synthesis, synthesis of nitroglycerin). The difference between the resulting molecular structures of nitro compounds and nitrates () is that the nitrogen atom in nitro compounds is directly Chemical bond, bonded to a non-oxygen atom (typically carbon or another nitrogen atom), whereas in nitrate esters (also called organic nitrates), the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom (nitrito group). There are many major industrial applications of nitration in the strict sense; the most important by volume are for the production of nitroaromatic compounds such as nitrobenzene. The technology is long-standing and mature. : Nitration rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" or "salt maker". When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room temperature the same becomes true of groups 1 and 15, assuming white phosphorus is taken as the standard state.This could also be the case for group 12, al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a characteristic pungent odor. Acetone is miscibility, miscible with properties of water, water and serves as an important organic solvent in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and for production of methyl methacrylate and bisphenol A, which are precursors to widely used plastics.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in organic chemistry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a salt substitute for table salt (NaCl), a fertilizer, as a medication, in scientific applications, in domestic water softeners (as a substitute for sodium chloride salt), as a feedstock, and in food processing, where it may be known as E number additive E508. It occurs naturally as the mineral sylvite, which is named after salt's historical designations ''sal degistivum Sylvii'' and ''sal febrifugum Sylvii'', and in combination with sodium chloride as sylvinite. Uses Fertilizer The majority of the potassium chloride produced is used for making fertilizer, called potash, since the growth of many plants is limited by potassium availabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |