|
Azeotropic Distillation
In chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, ''azeotropic distillation'' usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous (e.g. producing two, immiscible liquid phases), such as the example below with the addition of benzene to water and ethanol. This practice of adding an entrainer which forms a separate phase is a specific sub-set of (industrial) Azeotrope#Azeotropy in fluid separation processes, azeotropic distillation methods, or combination thereof. In some senses, adding an entrainer is similar to extractive distillation. Material separation agent The addition of a material separation agent, such as benzene to an ethanol/water mixture, changes the molecular interactions and eliminates the azeotrope. Added in the liquid phase, the new component can alter the activity coefficient of various comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isooctane
2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale (the zero point is ''n''-heptane). It is an important component of gasoline, frequently used in relatively large proportions (around 10%) to increase the knock resistance of fuel. Strictly speaking, if the standard meaning of ‘iso’ is followed, the name ''isooctane'' should be reserved for the isomer 2-methylheptane. However, 2,2,4-trimethylpentane is by far the most important isomer of octane and historically it has been assigned this name. Production Isooctane is produced on a massive scale in the petroleum industry by alkylation of isobutene with isobutane. This process is conducted in alkylation units in the presence of acid catalysts. : It can also be produced from isobutylene by dimerization using an Amberlyst cata ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theoretical Plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process. Applications The concept of theoretical plates and trays or equilibrium stages is used in the design of many different types of separation. Distillation columns The concept of theoretical plates in designing distillation processes has been discussed in many reference texts. Any physical device that provides good contact between the vapor and liquid phases pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
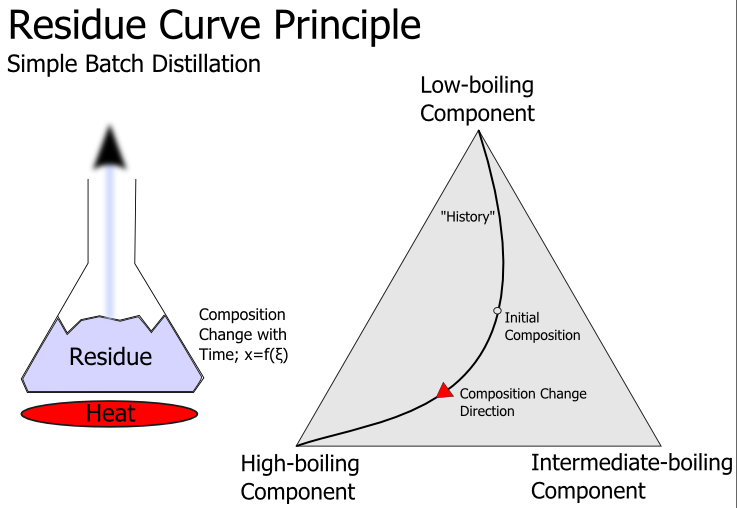
Residue Curve
A residue curve describes the change in the composition of the liquid phase of a chemical mixture during continuous evaporation at the condition of vapor–liquid equilibrium (open distillation). Multiple residue curves for a single system are called ''residue curves map''. Residue curves allow testing the feasibility of a separation of mixtures and therefore are a valuable tool in designing distillation processes. Residue curve maps are typically used for examining ternary mixtures which can't be easily separated by distillation because of Azeotrope, azeotropic points or too small relative volatility, relative volatilities. Characteristics # Residue curves start at the composition of a feed and then move to pure components or azeotropic points with higher temperatures (isobaric condition) or lower vapor pressures (isothermal condition). This happens because more of the light boiling substances are vaporized than of the high boiling substances and therefore the concentration of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azeotrope Tables
This page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The data include the composition of a mixture by weight (in binary azeotropes, when only one fraction is given, it is the fraction of the second component), the boiling point (b.p.) of a component, the boiling point of a mixture, and the specific gravity of the mixture. Boiling points are reported at a pressure of 760 mm Hg unless otherwise stated. Where the mixture separates into layers, values are shown for upper (U) and lower (L) layers. The data were obtained from Lange's 10th edition''Lange's Handbook of Chemistry'', 10th ed. pp. 1496–1505 and ''CRC Handbook of Chemistry and Physics'' 44th edition''CRC Handbook of Chemistry and Physics'', 44th ed. pp. 2143–2184 unless otherwise noted (see color code table). A list of 15825 binary and ternary mixtures was collated and published by the American Chemical Society. An azeotrope databank is also available online through the Uni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
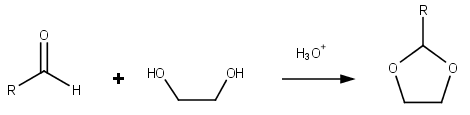
Dioxolane
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals. As a class of compounds Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol. (+)-''cis''-Dioxolane is the trivial name for which is a muscarinic acetylcholine receptor agonist. Protecting groups Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeolite
Zeolites are a group of several microporous, crystalline aluminosilicate minerals commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. The term was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material ''zeolite'', from the Greek , meaning "to boil" and , meaning "stone". Zeolites occur naturally, but are also produced industrially on a large scale. , 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained is examined by the International Zeolite Association Structure Commission (IZA-SC) and receives a three-letter designation. Character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rectified Spirit
Rectified spirit, also known as neutral spirits, rectified alcohol or ethyl alcohol of agricultural origin, is highly concentrated ethanol that has been purified by means of repeated distillation in a process called rectification. In some countries, denatured alcohol or denatured rectified spirit may commonly be available as "rectified spirit", because in some countries (though not necessarily the same) the retail sale of rectified alcohol in its non-denatured form is prohibited. The purity of rectified spirit has a practical limit of 97.2% ABV (95.6% by mass) when produced using conventional distillation processes, as a mixture of ethanol and water becomes a minimum-boiling azeotrope at this concentration. However, rectified spirit is typically distilled in continuous multi-column stills at 96–96.5% ABV and diluted as necessary. Ethanol is a commonly used medical alcohol''spiritus fortis'' is a medical term for ethanol solutions with 95% ABV. Neutral spirits can be produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Oven
In chemistry, a vacuum oven is an oven that can also apply a vacuum to its contents. Such devices are useful for removing solvent or dehydrating samples. They are equivalent to Abderhalden's drying pistol in some ways, but vacuum ovens typically can accommodate large samples. A characteristic operation for a vacuum oven is the activation or regeneration of molecular sieves. Vacuum furnaces are related devices that operate at much higher temperatures and much lower pressures. The acetylacetonate complex Mn(acac)2 is obtained by vacuum drying the yellow dihydrate Mn(acac)2(H2O)2.{{cite book , doi=10.1002/9780470132371.ch51 , chapter=Manganese(II) Acetylacetonate , date=1960 , last1=Charles , first1=Robert G. , title=Inorganic Syntheses , volume=6 , pages=164–166 , isbn=978-0-470-13165-7 References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Sieve
A molecular sieve is a material with pores of uniform size comparable to that of individual molecules, linking the interior of the solid to its exterior. These materials embody the molecular sieve effect, in which molecules larger than the pores are preferentially sieved, allowing for the selective adsorption of specific compounds based on their molecular size. Many kinds of materials exhibit some molecular sieves, but zeolites dominate the field. Zeolites are almost always aluminosilicates, or variants where some or all of the Si or Al centers are replaced by similarly charged elements. Sieving process The diameters of the pores that comprise molecular sieves are similar in size to small molecules. Large molecules cannot enter or be adsorbed, while smaller molecules can. As a mixture of molecules migrates through the stationary bed of porous, semi-solid substance referred to as a sieve (or matrix), the components of the highest molecular weight (which are unable to pass int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity Coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |