|
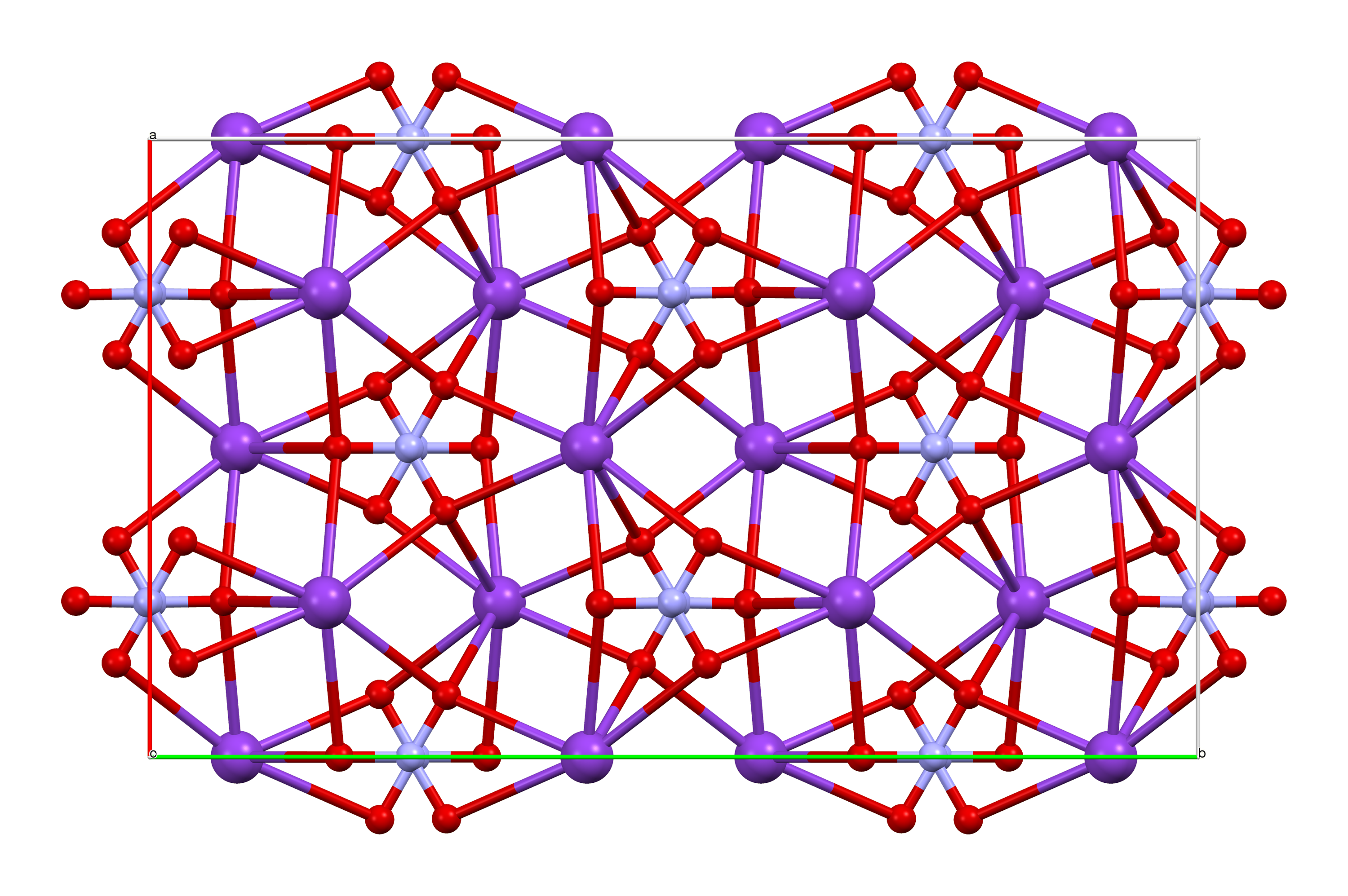
Arsenic Pentoxide
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications. Structure The structure consists of tetrahedral and octahedral centers linked by sharing corners. The structure differs from that of the corresponding Phosphorus pentoxide, phosphorus(V) oxide; as a result, although there is still a solid solution with that oxide, it only progresses to the equimolar point, at which point phosphorus has substituted for arsenic in all of its tetrahedral sites. Likewise, arsenic pentoxide can also dissolve up to an equimolar amount of antimony pentoxide, as antimony substitutes for arsenic only in its octahedral sites. Synthesis Historical Pierre Macquer found a crystallizable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopic
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption (chemistry), absorption or adsorption from the surrounding Natural environment, environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g. changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they dissolve in the water they absorb, forming an aqueous solution. Hygroscopy is essential for many plant and animal species' attainment of hydration, nutrition, reproduction and/or seed dispersal. Biological evolution created hygroscopic solutions for water harvesting, filament tensile strength, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Nitrate
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or ''nitre'' outside the United States). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpetre (or saltpeter in the United States). Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of traditional gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color. Etymology Nitre, or potassium nitrate, because of its early and global use and production, has many names. As for nitrate, Egyptian and Hebrew words for it had the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic(V) Compounds
Arsenic is a chemical element; it has symbol As and atomic number 33. It is a metalloid and one of the pnictogens, and therefore shares many properties with its group 15 neighbors phosphorus and antimony. Arsenic is notoriously toxic. It occurs naturally in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. It has various allotropes, but only the grey form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is also a common n-type dopant in semiconductor electronic devices, and a component of the III–V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the persistent toxicity of arsenic and its compounds. Arsen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Government Publishing Office
The United States Government Publishing Office (USGPO or GPO), formerly the United States Government Printing Office, is an agency of the legislative branch of the United States federal government. The office produces and distributes information products and services for all three branches of the Federal Government, including U.S. passports for the Department of State as well as the official publications of the Supreme Court, the Congress, the Executive Office of the President, executive departments, and independent agencies. An act of Congress changed the office's name to its current form in 2014. History Establishment of the Government Printing Office The Government Printing Office was created by congressional joint resolution () on June 23, 1860. It began operations March 4, 1861, with 350 employees and reached a peak employment of 8,500 in 1972. The agency began transformation to computer technology in the 1980s; along with the gradual replacement of paper with el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emergency Planning And Community Right-to-Know Act
The Emergency Planning and Community Right-to-Know Act of 1986 is a United States federal law passed by the 99th United States Congress located at Title 42, Chapter 116 of the U.S. Code, concerned with emergency response preparedness. On October 17, 1986, President Ronald Reagan signed into law the Superfund, Superfund Amendments and Reauthorization Act of 1986 (SARA). This act amended the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA), commonly known as Superfund. A free-standing law, the Emergency Planning and Community Right-to-Know Act of 1986 (EPCRA) was commonly known as SARA Title III. Its purpose is to encourage and support emergency planning efforts at the state and local levels and to provide the public and local governments with information concerning potential chemical hazards present in their communities. Background During the early morning hours of December 3, 1984, a Union Carbide plant in a village just South of Bhopal, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Extremely Hazardous Substances
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (). The list can be found as an appendix to . Updates as of 2006 can be seen on the Federal Register, (August 16, 2006). The data were provided by the United States Environmental Protection Agency (EPA). __NOTOC__ A * Acetone cyanohydrin * Acetone thiosemicarbazide * Acrolein * Acrylamide * Acrylonitrile * Acryloyl chloride * Adiponitrile * Aldicarb * Aldrin * Allyl alcohol * Allylamine * Aluminum phosphide * Aminopterin * Amiton * Amiton oxalate * Ammonia * Amphetamine * Aniline * Aniline, 2,4,6-trimethyl- * Antimony pentafluoride * Antimycin A * ANTU ( Alpha-Naphthylthiourea) * Arsenic pentoxide * Arsenous oxide * Arsenous trichloride * Arsine * Azinphos-ethyl * Azinphos-methyl B * Benzal chloride * Benzenamine, 3-(trifluoromethyl)- * Benzenearsonic acid * Benzimidazole, 4,5-dichloro-2-(trifluoromethyl)- * B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. LCysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems. Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Ancient Greek, Greek κύστις ''kýstis'', "bladder". The thiol is susceptible to oxidation to give the disulfide bond, disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. The deprotonated form can generally be described by the symbol Cym as well. When used as a food additive, cysteine has the E number E920. Cysteine is Genetic code, encoded by the codo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orpiment
Orpiment, also known as ″yellow arsenic blende″ is a deep-colored, orange-yellow arsenic sulfide mineral with formula . It is found in volcanic fumaroles, low-temperature hydrothermal veins, and hot springs and may be formed through sublimation (phase transition), sublimation. Orpiment takes its name from the Latin ''auripigmentum'' (''aurum'', "gold" + ''pigmentum'', "pigment"), due to its deep-yellow color. Orpiment once was widely used in artworks, medicine, and other applications. Because of its toxicity and instability, its usage has declined. Etymology The Latin ''auripigmentum'' (''aurum'', "gold" + ''pigmentum'', "pigment") referred both to its deep-yellow color and to the historical belief that it was thought to contain gold. The Latin term was used by Pliny the Elder, Pliny in the first century CE. The Greek for orpiment was ''arsenikon'', deriving from the Greek word ''arsenikos'', meaning "male", from the belief that metals were of different sexes. This Greek te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or "high-test peroxide", decomposes explosively when heated and has been used as both a monopropellant and an oxidizer in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly into water and elemental oxygen when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a Stabilizer (chemistry), stabilizer in a weakly acidic solution in an opaque bottle. Hydrogen peroxide is found in biological systems including the human body. Enzymes that u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 chemical structure, structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetism, diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agents
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scheele
Scheele is a surname of Germanic origin. Notable people with the surname include: *Carl Wilhelm Scheele (1742–1786), German-Swedish pharmaceutical chemist *George Heinrich Adolf Scheele (1808–1864), German botanist * Karin Scheele (b. 1968), Austrian politician; member of the European Parliament since 1999 * Leonard A. Scheele (1907–1993), American physician; Surgeon General of the United States, 1948-56 *Nick Scheele Sir Nicholas Vernon Scheele Order of St Michael and St George, KCMG (3 January 1944 – 18 July 2014) was an English business executive who served as president, from 2001–05, and Chief Operating Officer (COO), from 2001–04, of the Ford Motor ... (1944–2014), British businessman; chief operating officer of Ford Motor Company * Thomas von Scheele (b. 1969), Swedish table tennis player See also * Scheele (crater), the lunar impact crater {{surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |