|
Arbutin
β-Arbutin, also known by its International Nomenclature of Cosmetic Ingredients (INCI) name, arbutin, is a glycosylated derivative of hydroquinone. β-Arbutin is naturally present in the leaves and bark of a variety of plants, notably the bearberry plant, ''Arctostaphylos uva-ursi''. Utilized as a biosynthetic active ingredient in topical treatments for skin lightening, β-arbutin is aimed at addressing hyperpigmentation issues. Its mechanism of action involves inhibiting the activity of tyrosinase, an essential enzyme for melanin synthesis in the human skin, thereby leading to a reduction in hyperpigmentation. It is important to distinguish β-arbutin from its structurally similar stereoisomer, α-arbutin, which exhibits similar effects in clinical applications. Properties Arbutin is a compound where a glucose molecule, specifically -glucose, is chemically bound to hydroquinone. In aqueous solutions, glucose can exist in one of three stereoisomeric forms: α, β, or γ, wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arctostaphylos Uva-ursi
''Arctostaphylos uva-ursi'' is a plant species of the genus ''Arctostaphylos'' widely distributed across circumboreal regions of the subarctic Northern Hemisphere. Kinnikinnick (from the Unami language for "smoking mixture") is a common name in Canada and the United States. Growing up to in height, the leaves are evergreen. The flowers are white to pink and the fruit is a red berry. One of several related species referred to as bearberry, its specific epithet ''uva-ursi'' means "grape of the bear" in Latin, similar to the meaning of the generic epithet ''Arctostaphylos'' (Greek for "bear grapes"). Description ''Arctostaphylos uva-ursi'' is a small procumbent woody groundcover shrub growing to high. Wild stands of the species can be dense, with heights rarely taller than . Erect branching twigs emerge from long flexible prostrate stems, which are produced by single roots. The trailing stems will layer, sending out small roots periodically. The finely textured velvety branches ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
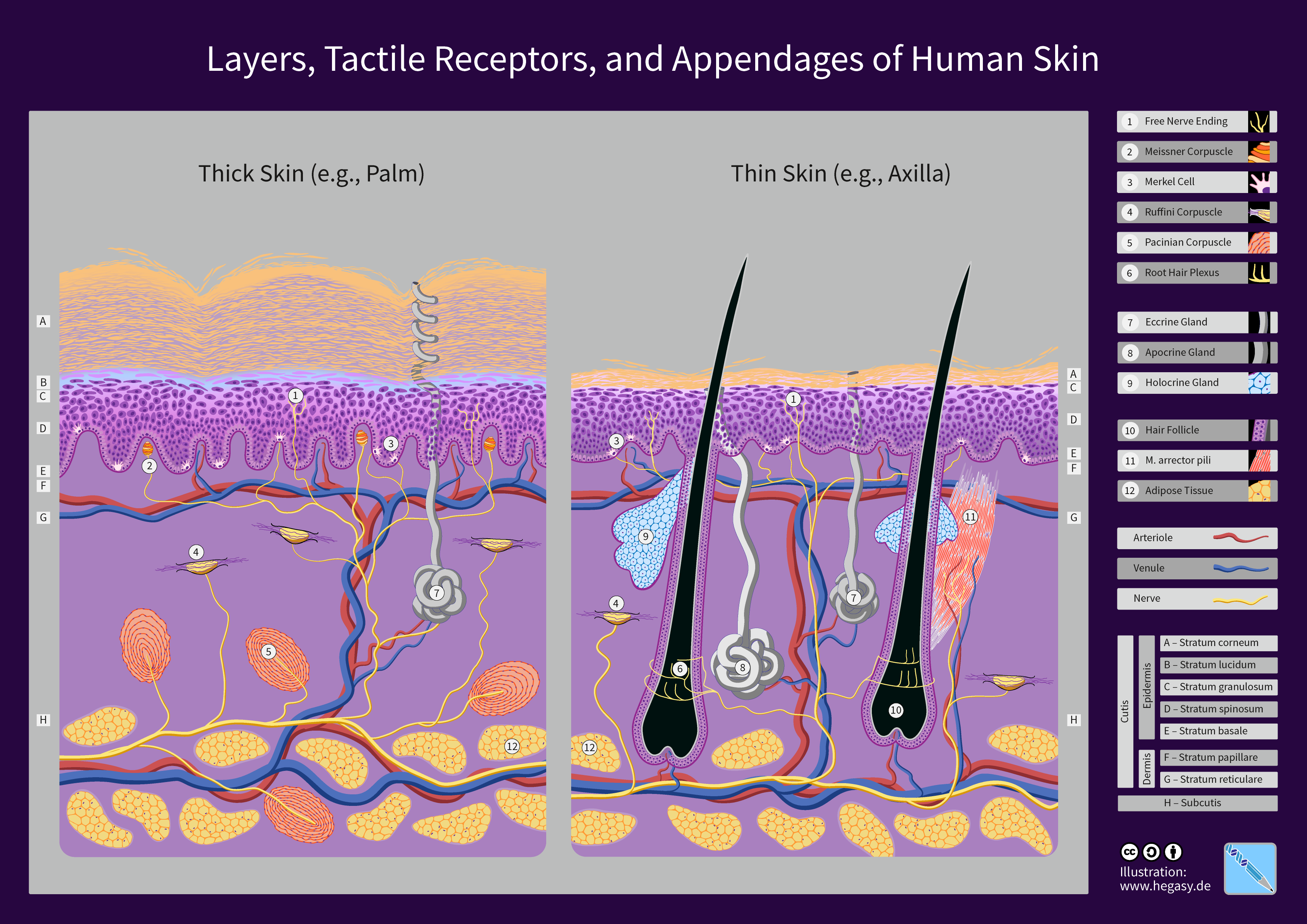
Human Skin
The human skin is the outer covering of the body and is the largest organ of the integumentary system. The skin has up to seven layers of ectodermal tissue (biology), tissue guarding Skeletal muscle, muscles, bones, ligaments and organ (anatomy), internal organs. Human skin is similar to most of the other mammals' skin, and it is very similar to pig skin. Though nearly all human skin is covered with hair follicles, it can appear Nudity#Evolution of hairlessness, hairless. There are two general types of skin: hairy and glabrous skin (hairless). The adjective List of medical roots, suffixes and prefixes#C, ''cutaneous'' literally means "of the skin" (from Latin ''cutis'', skin). Skin plays an important immunity (medical), immunity role in protecting the body against pathogens and excessive transepidermal water loss, water loss. Its other functions are Thermal insulation, insulation, thermoregulation, temperature regulation, sensation, synthesis of vitamin D, and the protection ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
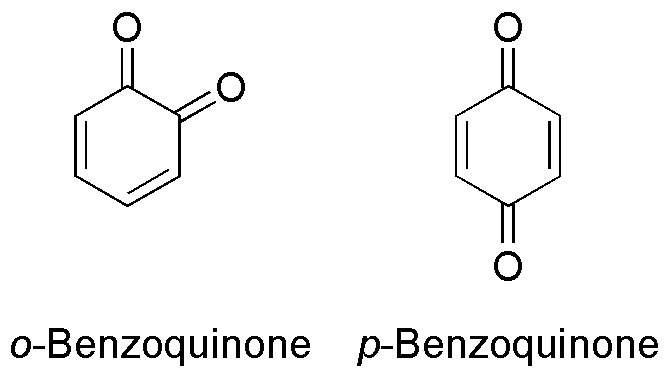
Benzoquinone
Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones: * 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone) * 1,2-Benzoquinone, less commonly, left image (also ''ortho''-benzoquinone, ''o''-benzoquinone, ''ortho''-quinone) *1,3-benzoquinone "does not exist, because its structure would be nonplanar and highly strained", though derivatives are known. An alkylated ''p''-benzoquinone has been found in the rhizomes of '' Iris kemaonensis''. See also * Arene substitution pattern Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * ... References {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotation (geometry), rotations, translation (geometry), translations, and some Conformational isomerism, conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physics, physical properties, except that they often have opposite optical activity, optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic mixture, racem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G/mol
In chemistry, the molar mass () (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical substance ( element or compound) is defined as the ratio between the mass () and the amount of substance (, measured in moles) of any sample of the substance: . The molar mass is a bulk, not molecular, property of a substance. The molar mass is a ''weighted'' ''average'' of many instances of the element or compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molecular mass (for molecular compounds) and formula mass (for non-molecular compounds, such as ionic salts) are commonly used as synonyms of molar mass, as the numerical values are identical (for all practical purposes), differing only in units ( dalton vs. g/mol or k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anomer
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers or diastereomers that differ at only the anomeric carbon, the carbon atom that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order for anomers to exist, the sugar must be in its cyclic form, since in open-chain form, the anomeric carbon atom is planar and thus achiral. More formally stated, then, an anomer is an epimer at the hemiacetal/hemiketal carbon atom in a cyclic saccharide. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations. Nomenclature Every two anomers are designated alpha (α) or beta (β), according to the configurational relationship between the ''anomeric centre'' and the ''anomeric reference atom'', hence they are relative stereodescriptors. The anomeric centre in hemiac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |