|
Alamethicin Synthase
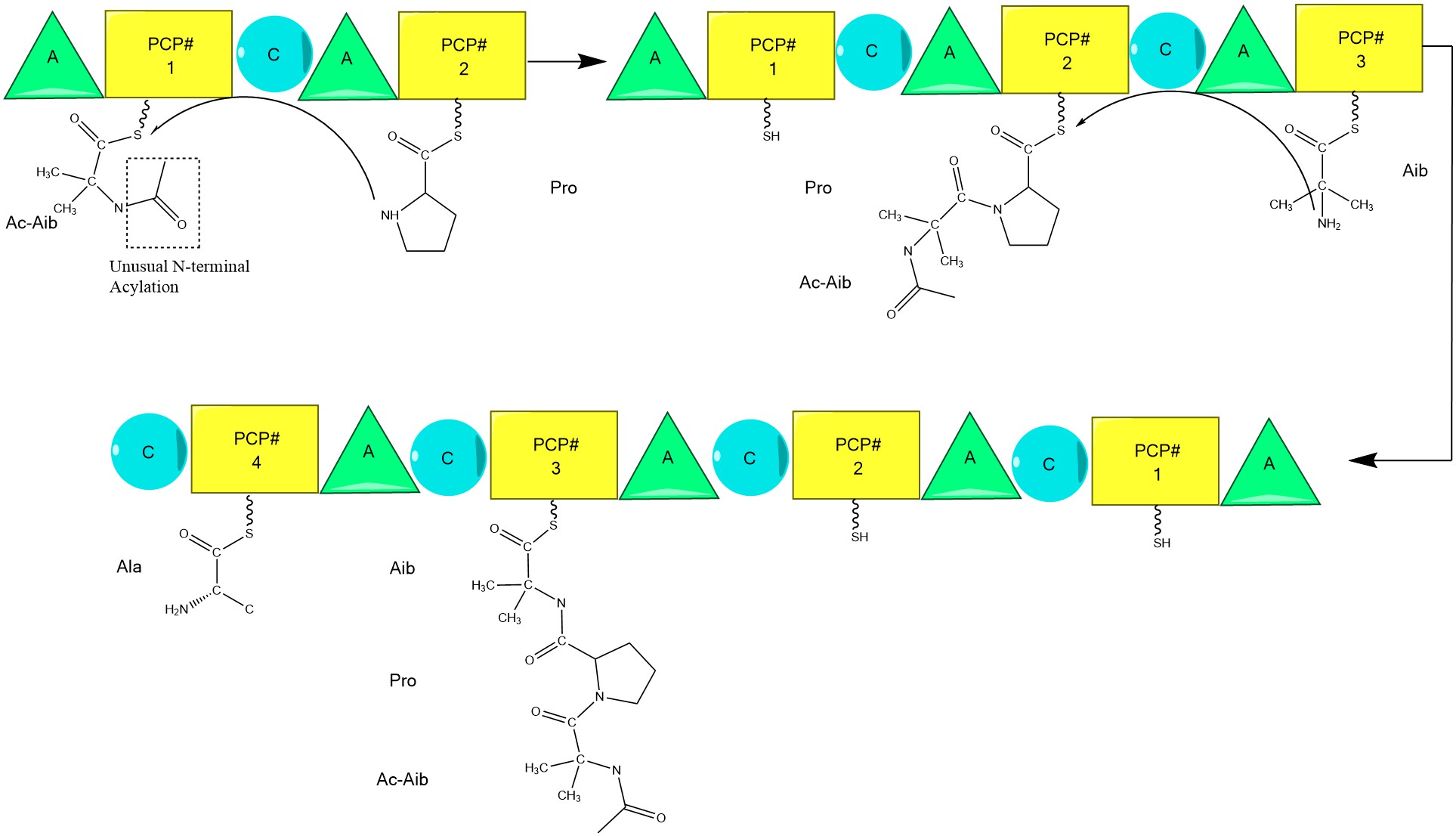
Alamethicin is a channel-forming peptide antibiotic, produced by the fungus ''Trichoderma viride''. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib (2-aminoisobutyric acid). This residue strongly induces formation of alpha-helical structure. The peptide sequence is : Ac-Aib-Pro-Aib-Ala-Aib-Ala-Gln-Aib-Val-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu-Gln-Phl where Ac = acetyl, Phl = phenylalaninol, and Aib = 2-Aminoisobutyric acid. In cell membranes, it forms voltage-dependent ion channels by aggregation of four to six molecules. Biosynthesis Alamethicin biosynthesis is hypothesized to be catalyzed by alamethicin synthase, a Nonribosomal peptide synthase (NRPS) first isolated in 1975. Although there are several sequences of the alamethicin peptide accepted, evidence suggests these all follow the general NRPS mechanism with small variations at select amino acids. Beginning with the acylation of the N terminal of the first aminoisobutiric a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermentek
Fermentek Ltd. is a biotechnological company in the Atarot industrial zone of Jerusalem, Israel. It specializes in the research, development and manufacture of biologically active, natural products isolated from microorganisms as well as from other natural sources such as plants and algae. The main microorganisms used are nonpathogenic actinomycetes, Nocardia and Streptomycetes. The fungi used are: Penicillium, Aspergillus, Fusarium and the like. None of these is a human pathogen. Fermentek does not sell to individuals. Most of its products are marketed through major international distributors specializing in chemicals, under their own brand names. Nevertheless, Fermentek has specific impact on the biochemical market, especially in the field of mycotoxins. Mycotoxins are toxic compounds produced by molds in human food and farm animal feeds, thus being economically important factors. Fermentek manufactures an extensive line of pure mycotoxins used as standards in food analysis. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), work needed per unit of Electric charge, charge to move a positive Test particle#Electrostatics, test charge from the first point to the second point. In the SI unit, International System of Units (SI), the SI derived unit, derived unit for voltage is the ''volt'' (''V''). The voltage between points can be caused by the build-up of electric charge (e.g., a capacitor), and from an electromotive force (e.g., electromagnetic induction in a Electric generator, generator). On a macroscopic scale, a potential difference can be caused by electrochemical processes (e.g., cells and batteries), the pressure-induced piezoelectric effect, and the thermoelectric effect. Since it is the difference in electric potential, it is a physical Scalar (physics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules such as proteins and nucleic acids, which is overseen by the Worldwide Protein Data Bank (wwPDB). This structural data is obtained and deposited by biologists and biochemists worldwide through the use of experimental methodologies such as X-ray crystallography, Nuclear magnetic resonance spectroscopy of proteins, NMR spectroscopy, and, increasingly, cryo-electron microscopy. All submitted data are reviewed by expert Biocuration, biocurators and, once approved, are made freely available on the Internet under the CC0 Public Domain Dedication. Global access to the data is provided by the websites of the wwPDB member organizations (PDBe, PDBj, RCSB PDB, and BMRB). The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NRPS Basics Corrected2
NRPS may refer to: * New Riders of the Purple Sage * Niagara Regional Police Service The Niagara Regional Police Service (NRPS) is a Regional police#Canada, regional police service maintained by the Regional Municipality of Niagara in the Canadian province of Ontario. As of 2021, the force employed 774 sworn police officers and 3 ... * Non Regular Permanent Staff * Non-Ribosomal Peptide Synthetase {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Carrier Protein
The acyl carrier protein (ACP) is a cofactor of both fatty acid and polyketide biosynthesis machinery. It is one of the most abundant proteins in cells of ''E. coli.'' In both cases, the growing chain is bound to the ACP via a thioester derived from the distal thiol of a 4'-phosphopantetheine moiety. Structure The ACPs are small negatively charged α-helical bundle proteins with a high degree of structural and amino acid similarity. The structures of a number of acyl carrier proteins have been solved using various NMR and crystallography techniques. The ACPs are related in structure and mechanism to the peptidyl carrier proteins (PCP) from nonribosomal peptide synthases. Biosynthesis Subsequent to the expression of the inactive ''apo'' ACP, the 4'-phosphopantetheine moiety is attached to a serine residue. This coupling is mediated by acyl carrier protein synthase (ACPS), a 4'-phosphopantetheinyl transferase. 4'-Phosphopantetheine is a prosthetic group of several acyl carrier pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
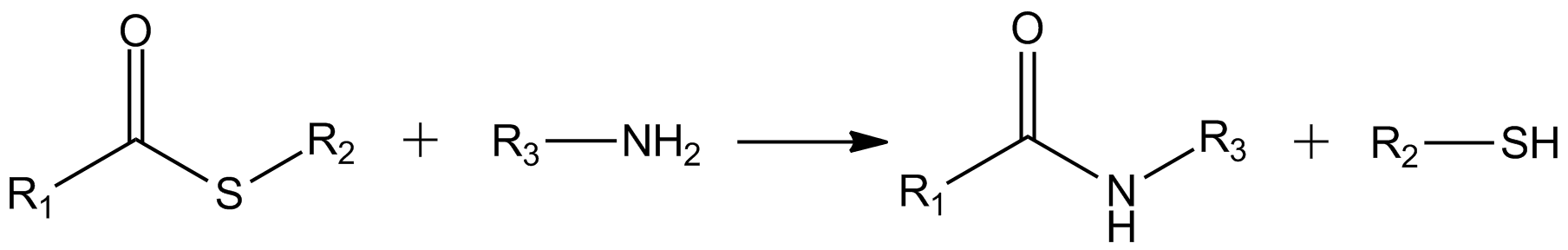
Thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid () with a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. The R and R' represent organyl groups, or H in the case of R. Synthesis One route to thioesters involves the reaction of an acid chloride with an alkali metal salt of a thiol: : Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: : Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenylylation
Adenylylation, more commonly known as AMPylation, is a process in which an adenosine monophosphate (AMP) molecule is covalently attached to the amino acid side chain of a protein. This covalent addition of AMP to a hydroxyl side chain of the protein is a post-translational modification. Adenylylation involves a phosphodiester bond between a hydroxyl group of the molecule undergoing adenylylation, and the phosphate group of the adenosine monophosphate nucleotide (i.e. adenylic acid). Enzymes that are capable of catalyzing this process are called AMPylators. The known amino acids to be targeted in the protein are tyrosine and threonine, and sometimes serine. When charges on a protein undergo a change, it affects the characteristics of the protein, normally by altering its shape via interactions of the amino acids which make up the protein. AMPylation can have various effects on the protein. These are properties of the protein like, stability, enzymatic activity, co-factor binding, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidation, oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a cysteamine, β-mercaptoethylamine group linked to pantothenic acid (vitamin B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through Beta oxidation, β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acylation
In chemistry, acylation is a broad class of chemical reactions in which an acyl group () is added to a substrate. The compound providing the acyl group is called the acylating agent. The substrate to be acylated and the product include the following: *alcohols, esters * amines, amides * arenes or alkenes, ketones A particularly common type of acylation is acetylation, the addition of the acetyl group. Closely related to acylation is formylation, which employ sources of "HCO+ in place of "RCO+". Examples Because they form a strong electrophile when treated with Lewis acids, acyl halides are commonly used as acylating agents. For example, Friedel–Crafts acylation uses acetyl chloride () as the agent and aluminum chloride () as a catalyst to add an acetyl group to benzene: This reaction is an example of electrophilic aromatic substitution. Acyl halides and acid anhydrides of carboxylic acids are also common acylating agents. In some cases, active esters exhibit compa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonribosomal Peptide
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacterium, bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria commensalism, inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term ''nonribosomal peptide'' typically refers to a very specific set of these as discussed in this article. Nonribosomal peptides are synthesized by nonribosomal peptide synthetases, which, unlike the ribosomes, are independent of messenger RNA. Each nonribosomal peptide synthetase can synthesize only one type of peptide. Nonribosomal peptides often have cyclic compound, cyclic and/or branched structures, can contain non-proteinogenic amino acids including D-amino acids, carry modifications like ''Nitrogen, N''-methyl and ''N''-formyl groups, or are Glycosylation, glycosylated, Acylation, acylated, Ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alamethicin Synthase
Alamethicin is a channel-forming peptide antibiotic, produced by the fungus ''Trichoderma viride''. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib (2-aminoisobutyric acid). This residue strongly induces formation of alpha-helical structure. The peptide sequence is : Ac-Aib-Pro-Aib-Ala-Aib-Ala-Gln-Aib-Val-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu-Gln-Phl where Ac = acetyl, Phl = phenylalaninol, and Aib = 2-Aminoisobutyric acid. In cell membranes, it forms voltage-dependent ion channels by aggregation of four to six molecules. Biosynthesis Alamethicin biosynthesis is hypothesized to be catalyzed by alamethicin synthase, a Nonribosomal peptide synthase (NRPS) first isolated in 1975. Although there are several sequences of the alamethicin peptide accepted, evidence suggests these all follow the general NRPS mechanism with small variations at select amino acids. Beginning with the acylation of the N terminal of the first aminoisobutiric a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |