|
Agmatine Deiminase
Agmatine, also known as 4-aminobutyl-guanidine, was discovered in 1910 by Albrecht Kossel. It is a chemical substance which is naturally created from the amino acid arginine. Agmatine has been shown to exert modulatory action at multiple molecular targets, notably: neurotransmitter systems, ion channels, nitric oxide (NO) synthesis, and polyamine metabolism and this provides bases for further research into potential pharmacological applications. History The term agmatine stems from A- (for amino-) + g- (from guanidine) + -ma- (from ptomaine) + -in (German)/-ine (English) suffix with insertion of -t- apparently for euphony. A year after its discovery, it was found that agmatine could increase blood flow in rabbits; however, the physiological relevance of these findings were questioned given the high concentrations (high μM range) required. In the 1920s, researchers in the diabetes clinic of Oskar Minkowski showed that agmatine can exert mild hypoglycemic effects. In 1994, endog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albrecht Kossel
Ludwig Karl Martin Leonhard Albrecht Kossel (; 16 September 1853 – 5 July 1927) was a biochemist and pioneer in the study of genetics. He was awarded the Nobel Prize for Physiology or Medicine in 1910 for his work in determining the chemical composition of nucleic acids, the genetic substance of biological cells. Kossel isolated and described the five organic compounds that are present in nucleic acid: adenine, cytosine, guanine, thymine, and uracil. These compounds were later shown to be nucleobases, and are key in the formation of DNA and RNA, the genetic material found in all living cells. Kossel was an important influence on and collaborator with other important researchers in biochemistry, including Henry Drysdale Dakin, Friedrich Miescher, Edwin B. Hart, and his professor and mentor, Felix Hoppe-Seyler. Kossel was editor of the Zeitschrift für Physiologische Chemie (Journal of Physiological Chemistry) from 1895 until his death. Kossel also conducted important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde Dehydrogenase
Aldehyde dehydrogenases () are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes (R–C(=O)) to carboxylic acids (R–C(=O)). The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes. Function Aldehyde dehydrogenase is a polymorphic enzyme responsible for the oxidation of aldehydes to carboxylic acids. There are three different classes of these enzymes in mammals: class 1 (low ''K''m, cytosolic), class 2 (low ''K''m, mitochondrial), and class 3 (high ''K''m, such as those expressed in tumors, stomach, and cornea). In all three classes, constitutive and inducible forms exist. ALDH1 and ALDH2 are the most important enzymes for aldehyde oxidation, and both are tetrameric enzymes composed of 54 kDa subunits. These enzymes are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
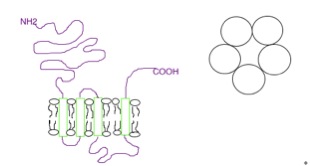
Membrane Transporter
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion, active transport, osmosis, or reverse diffusion. The two main types of proteins involved in such transport are broadly categorized as either ''channels'' or ''carriers'' (a.k.a. transporters, or permeases). Examples of channel/carrier proteins include the GLUT 1 uniporter, sodium channels, and potassium channels. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are known as the transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid-sensing Ion Channel
Acid-sensing ion channels (ASICs) are neuronal voltage-insensitive sodium channels activated by extracellular protons permeable to Na+. ASIC1 also shows low Ca2+ permeability. ASIC proteins are a subfamily of the ENaC/Deg superfamily of ion channels. These genes have splice variants that encode for several isoforms that are marked by a suffix. In mammals, acid-sensing ion channels (ASIC) are encoded by five genes that produce ASIC protein subunits: ASIC1, ASIC2, ASIC3, ASIC4, and ASIC5. Three of these protein subunits assemble to form the ASIC, which can combine into both homotrimeric and heterotrimeric channels typically found in both the central nervous system and peripheral nervous system. However, the most common ASICs are ASIC1a and ASIC1a/2a and ASIC3. ASIC2b is non-functional on its own but modulates channel activity when participating in heteromultimers and ASIC4 has no known function. On a broad scale, ASICs are potential drug targets due to their involvement in pathologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage-gated Ca2+ Channel
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (''e.g.'' muscle, glial cells, neurons) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+–Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions. At physiologic or resting membrane potential, VGCCs are normally closed. They are activated (''i.e.'': opened) at depolarized membrane potentials and this is the source of the "voltage-gated" epithet. The concentration of calcium (Ca2+ ions) is normally several thousand times higher outside the cell than inside. Activation of particular VGCCs allows a Ca2+ influx into the cell, which, depending on the cell type, results in activation of calcium-sensitive potassium channels, muscular contraction, excitation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATP-sensitive K+ Channel
An ATP-sensitive potassium channel (or KATP channel) is a type of potassium channel that is gated by intracellular nucleotides, ATP and ADP. ATP-sensitive potassium channels are composed of Kir6.x-type subunits and sulfonylurea receptor (SUR) subunits, along with additional components. KATP channels are widely distributed in plasma membranes; however some may also be found on subcellular membranes. These latter classes of KATP channels can be classified as being either sarcolemmal ("sarcKATP"), mitochondrial ("mitoKATP"), or nuclear ("nucKATP"). Discovery and structure KATP channels were first identified in cardiac myocytes by Akinori Noma in Japan. Glucose-regulated KATP channel activity was found in pancreatic beta cells by Frances Ashcroft at the University of Oxford. The closure of KATP channels leads to increased insulin secretion in beta cells and reduces glucagon secretion in alpha cells. SarcKATP are composed of eight protein subunits (octamer). Four of these a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by Gating (electrophysiology), gating the flow of ions across the cell membrane, controlling the flow of ions across secretion, secretory and epithelial cells, and regulating cell (biology), cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophore, ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5HT-3 Receptor
The 5-HT3 receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT receptors (5-hydroxytryptamine, or serotonin receptors) which are G protein-coupled receptors. This ion channel is cation-selective and mediates neuronal depolarization and excitation within the central and peripheral nervous systems. As with other ligand gated ion channels, the 5-HT3 receptor consists of five subunits arranged around a central ion conducting pore, which is permeable to sodium (Na), potassium (K), and calcium (Ca) ions. Binding of the neurotransmitter 5-hydroxytryptamine (serotonin) to the 5-HT3 receptor opens the channel, which, in turn, leads to an excitatory response in neurons. The rapidly activating, desensitizing, inward current is predominantly carried by sodium and potassium ions. 5-HT3 receptors have a negligible permeability to anions. They are most closely related by homology to the nicotinic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2A Receptor
The 5-HT2A receptor is a subtype of the 5-HT2 receptor, 5-HT2 receptor that belongs to the serotonin receptor family and functions as a GPCR, G protein-coupled receptor (GPCR). It is a cell surface receptor that activates multiple intracellular signalling cascades. Like all 5-HT2 receptors, the 5-HT2A receptor is coupled to the Gq protein, Gq/G11 signaling pathway. It is the primary excitatory receptor subtype among the serotonin-responsive GPCRs. The 5-HT2A receptor was initially noted for its central role as the primary target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. It later regained research prominence when found to mediate, at least in part, the effects of many antipsychotic drugs, particularly atypical antipsychotic, atypical antipsychotics. Downregulation of post-synaptic 5-HT2A receptors is an adaptive response triggered by chronic administration of selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics. Elevated 5-HT2A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in multiple tissues including the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin (i.e., 5-hydroxytryptamine, hence "5-HT") receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand. The serotonin receptors modulate the release of many neurotransmitters, including glutamate, GABA, dopamine, epinephrine / norepinephrine, and acetylcholine, as well as many hormones, including oxytocin, prolactin, vasopressin, cortisol, corticotropin, and substance P, among others. Serotonin receptors influence various biological and neurological processes such as aggression, anxiety, appetite, cognition, learning, memory, mood, nausea, sleep, and thermoregulation. They are the target of a variety of pharmaceutical and recreational drugs, inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-2 Adrenergic Receptor
The alpha-2 (α2) adrenergic receptor (or adrenoceptor) is a G protein-coupled receptor (GPCR) associated with the Gi alpha subunit, Gi heterotrimeric G-protein. It consists of three highly homologous subtypes, including α2A-adrenergic, α2A-, α2B-adrenergic, α2B-, and α2C-adrenergic, α2C-adrenergic. Some species other than humans express a fourth α2D-adrenergic receptor as well. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α2-adrenergic receptor in the central nervous system, central and peripheral nervous systems. Cellular localization The Alpha-2A adrenergic receptor, α2A adrenergic receptor is localised in the following central nervous system (CNS) structures: * Brainstem (especially the locus coeruleus as presynaptic & somatodendritic autoreceptor ) * Midbrain * Hypothalamus * Olfactory system * Hippocampus * Spinal cord * Cerebral cortex * Cerebellum * Septum Whereas the Alpha-2B adrenergic receptor, α2B adren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazoline Receptor
Imidazoline receptors are the primary receptors on which clonidine and other imidazolines act. There are three main classes of imidazoline receptor: I1 is involved in inhibition of the sympathetic nervous system to lower blood pressure, I2 has as yet uncertain functions but is implicated in several psychiatric conditions, and I3 regulates insulin secretion. Classes As of 2017, there are three known subtypes of imidazoline receptors: I1, I2, and I3. I1 receptor The I1 receptor appears to be a G protein-coupled receptor that is localized on the plasma membrane. It may be coupled to PLA2 signalling and thus prostaglandin synthesis. In addition, activation inhibits the sodium-hydrogen antiporter and enzymes of catecholamine synthesis are induced, suggesting that the I1 receptor may belong to the neurocytokine receptor family, since its signaling pathways are similar to those of interleukins. It is found in the neurons of the reticular formation, the dorsomedial medulla oblo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |