|
Acylation
In chemistry, acylation is a broad class of chemical reactions in which an acyl group () is added to a substrate. The compound providing the acyl group is called the acylating agent. The substrate to be acylated and the product include the following: *alcohols, esters * amines, amides * arenes or alkenes, ketones A particularly common type of acylation is acetylation, the addition of the acetyl group. Closely related to acylation is formylation, which employ sources of "HCO+ in place of "RCO+". Examples Because they form a strong electrophile when treated with Lewis acids, acyl halides are commonly used as acylating agents. For example, Friedel–Crafts acylation uses acetyl chloride () as the agent and aluminum chloride () as a catalyst to add an acetyl group to benzene: This reaction is an example of electrophilic aromatic substitution. Acyl halides and acid anhydrides of carboxylic acids are also common acylating agents. In some cases, active esters exhibit compa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Friedel–Crafts Reaction
The Friedel–Crafts reactions are a set of organic reaction, reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an Aromatic hydrocarbon, aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution. Alkylation With alkenes In commercial applications, the alkylating agents are generally alkenes, some of the largest scale reactions practiced in industry. Such alkylations are of major industrial importance, e.g. for the production of ethylbenzene, the precursor to polystyrene, from benzene and ethylene and for the production of cumene from benzene and propene in cumene process: : : Industrial production typically uses solid acids derived from a zeolite as the catalyst. With alkyl halides Friedel–Crafts alkylation involves the alkylation of an aromatic ring. Traditionally, the alkylating agents are alkyl halides. Many alkylating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aluminium Chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron(III) chloride, giving them a yellow colour. The anhydrous form is commercially important. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
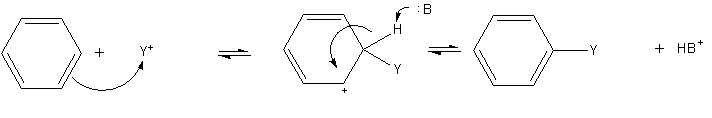
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, alkylation Friedel–Crafts reaction and acylation Friedel–Crafts reaction. Illustrative reactions The most widely practised example of this reaction is the ethylation of benzene. :: Approximately 24,700,000 tons were produced in 1999. (After dehydrogenation and polymerization, the commodity plastic polystyrene is produced.) In this process, acids are used as catalyst to generate the incipient carbocation. Many other electrophilic reactions of benzene are conducted, although on a much smaller scale; they are valuable routes to key intermediates. The nitration of benzene is achieved via the action of the nitronium ion as the electrophile. The Aromatic sulfonation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Clemmensen Reduction
Clemmensen reduction is a chemical reaction described as a Redox, reduction of ketones or aldehydes to alkanes using zinc Amalgam (chemistry), amalgam and concentrated hydrochloric acid (HCl). This reaction is named after Erik Christian Clemmensen, a Danish-American chemist. Clemmensen reduction conditions are particularly effective at reducing aryl-alkyl ketones, such as those formed in a Friedel-Crafts acylation. The two-step sequence of Friedel-Crafts acylation followed by Clemmensen reduction constitutes a classical strategy for the primary alkylation of arenes. Mechanism Despite the reaction being first discovered in 1914, the mechanism of the Clemmensen reduction remains obscure. Due to the Heterogeneous Reaction, heterogeneous nature of the reaction, mechanistic studies are difficult, and only a handful of studies have been disclosed. Mechanistic proposals generally invoke Organozinc chemistry, organozinc intermediates, sometimes including zinc carbenoids, either as d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Benzen Acylowany
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. Benzene is a volatile organic compound. Benzene is classified as a carcin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acyl Halide
An acyl halide (also known as an acid halide) is a chemical compound derived from an oxoacid by replacing a hydroxyl group () with a halide group (, where X is a halogen). In organic chemistry, the term typically refers to acyl halides of carboxylic acids (), which contain a functional group consisting of a carbonyl group () singly bonded to a halogen atom. The general formula for such an acyl halide can be written RCOX, where R may be, for example, an alkyl group, CO is the carbonyl group, and X represents the halide, such as chloride. Acyl chlorides are the most commonly encountered acyl halides, but acetyl iodide is the one produced (transiently) on the largest scale. Billions of kilograms are generated annually in the production of acetic acid. Preparation Aliphatic acyl halides On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid: : Common syntheses of acyl chlorides also entail the reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl group (). In organic chemistry, the acyl group (IUPAC name alkanoyl if the organyl group is alkyl) is usually derived from a carboxylic acid, in which case it has the formula , where R represents an organyl group or hydrogen. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond. Reactivity trends There are five main types of acyl derivatives. Acid halides are the most reactive towards nucleophiles, followed by anhydrides, esters, and amides. Carboxylate ions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acyl Halide
An acyl halide (also known as an acid halide) is a chemical compound derived from an oxoacid by replacing a hydroxyl group () with a halide group (, where X is a halogen). In organic chemistry, the term typically refers to acyl halides of carboxylic acids (), which contain a functional group consisting of a carbonyl group () singly bonded to a halogen atom. The general formula for such an acyl halide can be written RCOX, where R may be, for example, an alkyl group, CO is the carbonyl group, and X represents the halide, such as chloride. Acyl chlorides are the most commonly encountered acyl halides, but acetyl iodide is the one produced (transiently) on the largest scale. Billions of kilograms are generated annually in the production of acetic acid. Preparation Aliphatic acyl halides On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid: : Common syntheses of acyl chlorides also entail the reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nucleophilic Acyl Substitution
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl group (). In organic chemistry, the acyl group (IUPAC name alkanoyl if the organyl group is alkyl) is usually derived from a carboxylic acid, in which case it has the formula , where R represents an organyl group or hydrogen. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond. Reactivity trends There are five main types of acyl derivatives. Acid halides are the most reactive towards nucleophiles, followed by anhydrides, esters, and amides. Carboxylate ions are esse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Active Ester
In organic chemistry, an active ester is an ester functional group that is highly susceptible toward nucleophilic attack. Activation can be imparted by modifications of the acyl or the alkoxy components of a normal ester, say ethyl acetate. Typical modifications call for electronegative substituents. Active esters are employed in both synthetic and biological chemistry. Reactivity Active esters are mainly used as acylating agents. They undergo the same reactions as their unactivated analogues but do so more rapidly. They are prone to hydrolysis, for example. Of great interest is the enhanced reactivity of active esters toward amines to give amides. Examples Biochemistry 120px, 3'-Phosphoadenosine-5'-phosphosulfate is an active ester of sulfate. Active esters are prominent in biochemistry. Glutamine synthetase is an enzyme that forms an active ester from the terminal carboxylate of glutamic acid. This activation, imparted by phosphorylation, facilitates the conversion of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acetyl Chloride
Acetyl chloride () is an acyl chloride derived from acetic acid (). It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl. Synthesis On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid: : Laboratory routes Acetyl chloride was first prepared in 1852 by French chemist Charles Gerhardt by treating potassium acetate with phosphoryl chloride. Acetyl chloride is produced in the laboratory by the reaction of acetic acid with chlorodehydrating agents such as phosphorus trichloride (), phosphorus pentachloride (), sulfuryl chloride (), phosgene, or thionyl chloride (). However, these methods usually give acetyl chloride contaminated by phosphorus or sulfur impurities, which may interfere with the organic reactions. Other methods When heated, a mixture of dichloroacetyl chloride and acetic aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |