|
Abyssomicin
''Atrop''-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin. In 2006, the Nicolaou group discovered ''atrop''-abyssomicin C while working on the total synthesis of abyssomicin C. Then in 2007, Süssmuth and co-workers isolated ''atrop''-abyssomicin C from '' Verrucosispora maris'' AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that ''atrop''-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB ( 4-amino-4-deoxychorismate synthase), thereby depleting the biosynthesis of '' p'' -aminobenzoate. Structure ''Atrop''-abyssomicin C has a complex, yet intriguing structural topography. The compound contains an oxabicycl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Verrucosispora Maris
''Micromonospora maris'' is a Gram-positive bacterium from the genus ''Micromonospora'' which has been isolated from deep-sea sediments from the Sea of Japan The Sea of Japan is the marginal sea between the Japanese archipelago, Sakhalin, the Korean Peninsula, and the mainland of the Russian Far East. The Japanese archipelago separates the sea from the Pacific Ocean. Like the Mediterranean Sea, it h .... ''Verrucosispora maris'' produces abyssomicins and proximicins. References Further reading * * External linksType strain of ''Verrucosispora maris'' at Bac''Dive'' - the Bacterial Diversity Metadatabase Micromonosporaceae Bacteria described in 2012 {{Actinobacteria-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminodeoxychorismate Synthase
In enzymology, an aminodeoxychorismate synthaseEC 2.6.1.85 is an enzyme that catalyzes the chemical reaction :chorismate + L-glutamine \rightleftharpoons 4-amino-4-deoxychorismate + L-glutamate Thus, the two substrates of this enzyme are chorismate and L-glutamine, whereas its two products are 4-amino-4-deoxychorismate and L-glutamate. It is part of a pathway for the biosynthesis of ''para''-aminobenzoic acid (PABA); a precursor for the production of folates. Folates are family of cofactors that are essential for living organisms. Folate cofactors are used in several one-carbon transfer reactions required during the synthesis of essential metabolites, including methionine and thymidylate. Aminodeoxychorismate synthase (PabB), a 51 kDa protein in '' E. coli'', is encoded by the gene ''pabB''. 4-amino-4-deoxychorismate, the product of PabB, can be converted to ''para''-aminobenzoic acid by the enzyme 4-amino-4-deoxychorismate lyase (PabC). Nonmenclature This enzyme belon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
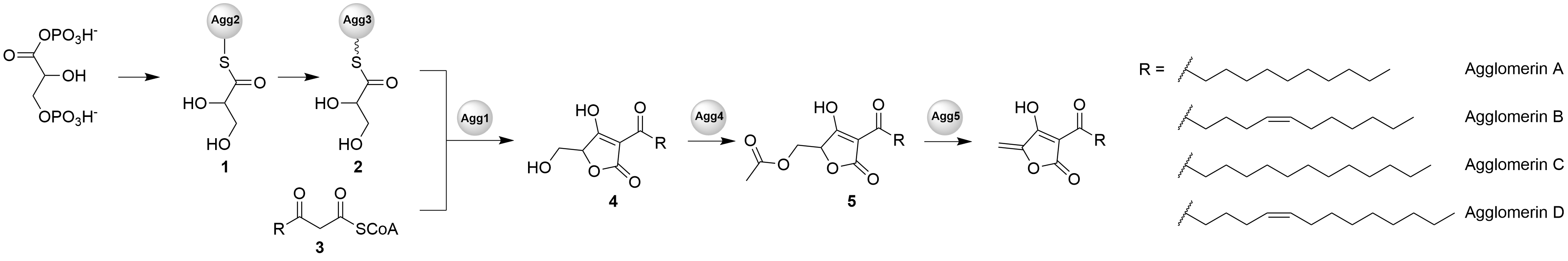
Agglomerin
Agglomerins are bacterial natural products, identified as metabolites of ''Pantoea agglomerans'' which was isolated in 1989 from river water in Kobe, Japan. They belong to the class of tetronate antibiotics, which include tetronomycin, tetronasin, and Atrop-abyssomicin C, abyssomicin C. The members of the agglomerins differ only in the composition of the acyl chain attached to the tetronate ring. They possess antibiotic activity against anaerobic bacteria and weak activity against aerobic bacteria ''in vitro''. The structures were solved in 1990. Agglomerin A is the major component (38%), followed by agglomerin B (30%), agglomerin C (24%), and agglomerin D (8%). Biosynthesis The biosynthetic gene cluster for agglomerins is 12 kb, and codes for 7 open reading frames. The glyceryl-S-ACP is derived from D-1,3-bisphosphoglycerate by Agg2 (glyceryl-''S''-ACP synthase) and Agg3 (acyl carrier protein). The acyl chain is taken from primary metabolism as a 3-oxoacyl-CoA thioester. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactones
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Lactones are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids, which takes place spontaneously when the ring that is formed is five- or six-membered. Lactones with three- or four-membered rings (α-lactones and β-lactones) are very reactive, making their isolation difficult. Special methods are normally required for the laboratory synthesis of small-ring lactones as well as those that contain rings larger than six-membered. Nomenclature Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterials (such as sulfonamides and antise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis Of Atrop Abyssomycin C
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g. NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atropisomer
Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers. They occur naturally and are important in pharmaceutical design. When the substituents are achiral, these conformers are enantiomers (''atropoenantiomers''), showing axial chirality; otherwise they are diastereomers (''atropodiastereomers''). Etymology and history The word ''atropisomer'' ( el, άτροπος, , meaning "without turn") was coined in application to a theoretical concept by German biochemist Richard Kuhn for Karl Freudenberg's seminal ''Stereochemie'' volume in 1933. Atropisomerism was first experimentally detected in a tetra substituted biphenyl, a diacid, by George Christie and James Kenner in 1922. Michinori Ōki further refined the definition of atropisomers taking into account the temperature-dependence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Aminobenzoic Acid
4-Aminobenzoic acid (also known as ''para''-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the ''para'' position) is an organic compound with the formula H2NC6H4CO2H. PABA is a white solid, although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino and carboxyl groups. The compound occurs extensively in the natural world. Production and occurrence In industry, PABA is prepared mainly by two routes: * Reduction of 4-nitrobenzoic acid * Hoffman degradation of the monoamide derived from terephthalic acid. Food sources of PABA include liver, brewer's yeast (and unfiltered beer), kidney, molasses, mushrooms, and whole grains. A review on this compound. Biology Biochemistry PABA is an intermediate in the synthesis of folate by bacteria, plants, and fungi. Many bacteria, including those found in the human intestinal tract such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |