|
Zirconium Propionate
Zirconium propionate is an ill-defined compound containing propionate and zirconium(IV).Zirconium Propionate (PDF) It is not soluble in water, but dissolves in isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ... , ethanol and ethyl acetate. When ...
[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the Interna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Acetate
Ethyl acetate ( systematically ethyl ethanoate, commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. Production and synthesis Ethyl acetate was first synthesized by the Count de Lauraguais in 1759 by distilling a mixture of ethanol and acetic acid. In 2004, an estimated 1.3 million tonnes were produced worldwide. The combined annual production in 1985 of Japan, North America, and Europe was about 400,000 tonnes. The global ethyl acetate market was valued at $3.3 billion in 2018. Ethyl acetate is synthesized in industry mainly via the classic Fischer esterification reaction of ethanol and acetic acid. This mixture converts to the ester in ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adhesion
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another ( cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another). The forces that cause adhesion and cohesion can be divided into several types. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are also certain emergent mechanical effects. Surface energy Surface energy is conventionally defined as the work that is required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cleave a bulk sample, creating two surfaces. If the new surfaces are identical, the surface energy γ of each surface is equal to half the work of cleavage, W: γ = (1/2)W11. If the surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic Organic may refer to: * Organic, of or relating to an organism, a living entity * Organic, of or relating to an anatomical organ Chemistry * Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ... solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners ( toluene, turpentine); as nail polish removers and solvents of glue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
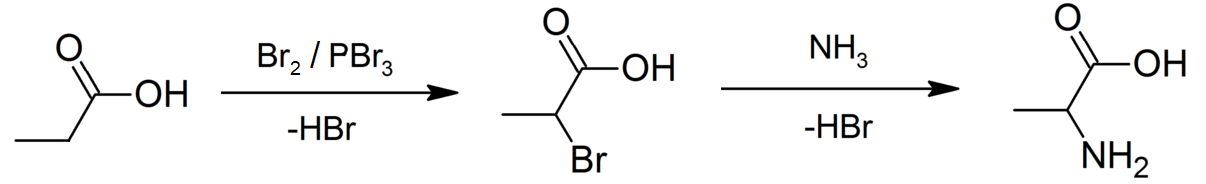
Propionates
Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH3CH2CO2− as well as the salts and esters of propionic acid are known as propionates or propanoates. History Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas established all the acids to be the same compound, which he called propionic acid, from the Greek words πρῶτος (prōtos), meaning ''first'', and πίων (piōn), meaning ''fat'', because it is the smallest H(CH2)''n''COOH acid that exhibits ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |