|
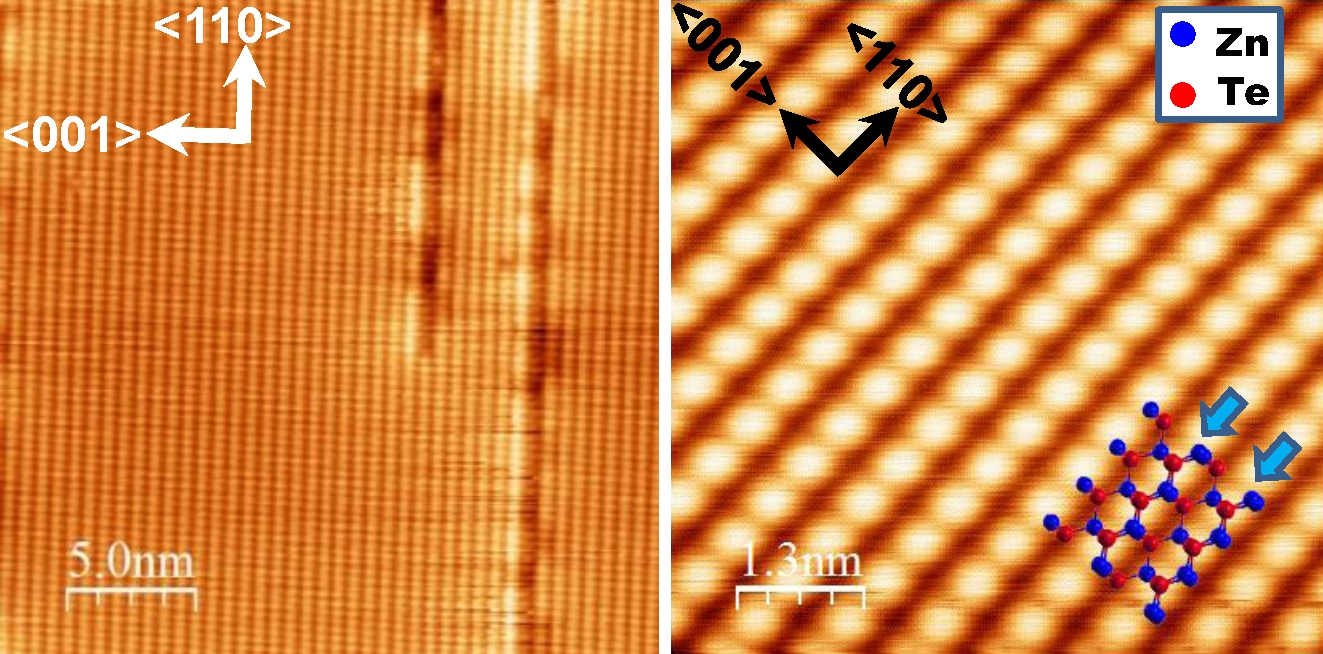
Zinc Telluride
Zinc telluride is a binary chemical compound with the formula ZnTe. This solid is a semiconductor material with a direct band gap of 2.26 eV. It is usually a p-type semiconductor. Its crystal structure is cubic, like that for sphalerite and diamond. Properties ZnTe has the appearance of grey or brownish-red powder, or ruby-red crystals when refined by sublimation. Zinc telluride typically has a cubic (sphalerite, or "zincblende") crystal structure, but can be also prepared as rocksalt crystals or in hexagonal crystals (wurtzite structure). Irradiated by a strong optical beam burns in presence of oxygen. Its lattice constant is 0.6101 nm, allowing it to be grown with or on aluminium antimonide, gallium antimonide, indium arsenide, and lead selenide. With some lattice mismatch, it can also be grown on other substrates such as GaAs, and it can be grown in thin-film polycrystalline (or nanocrystalline) form on substrates such as glass, for example, in the manufacture of thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zincblende
Sphalerite is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Valley type, and volcanogenic massive sulfide deposits. It is found in association with galena, chalcopyrite, pyrite (and other sulfides), calcite, dolomite, quartz, rhodochrosite, and fluorite. German geologist Ernst Friedrich Glocker discovered sphalerite in 1847, naming it based on the Greek word ''sphaleros'', meaning "deceiving", due to the difficulty of identifying the mineral. In addition to zinc, sphalerite is an ore of cadmium, gallium, germanium, and indium. Miners have been known to refer to sphalerite as ''zinc blende'', ''black-jack'', and ''ruby blende''. Marmatite is an opaque black variety with a high iron content. Crystal habit and structure Sphalerite crystallizes in the face-centered cubic zincblende crystal structure, which was named after the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doping (semiconductor)
In semiconductor production, doping is the intentional introduction of impurities into an intrinsic (undoped) semiconductor for the purpose of modulating its electrical, optical and structural properties. The doped material is referred to as an extrinsic semiconductor. Small numbers of dopant atoms can change the ability of a semiconductor to conduct electricity. When on the order of one dopant atom is added per 100 million intrinsic atoms, the doping is said to be ''low'' or ''light''. When many more dopant atoms are added, on the order of one per ten thousand atoms, the doping is referred to as ''high'' or ''heavy''. This is often shown as ''n+'' for n-type doping or ''p+'' for p-type doping. (''See the article on semiconductors for a more detailed description of the doping mechanism.'') A semiconductor doped to such high levels that it acts more like a conductor than a semiconductor is referred to as a degenerate semiconductor. A semiconductor can be considered i-typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Introduction To Solid State Physics
''Introduction to Solid State Physics'', known colloquially as ''Kittel'', is a classic condensed matter physics textbook written by American physicist Charles Kittel in 1953. The book has been highly influential and has seen widespread adoption; Marvin L. Cohen remarked in 2019 that Kittel's content choices in the original edition played a large role in defining the field of solid-state physics. It was also the first proper textbook covering this new field of physics. The book is published by John Wiley and Sons and, as of 2018, it is in its ninth edition and has been reprinted many times as well as translated into over a dozen languages, including Chinese, French, German, Hungarian, Indonesian, Italian, Japanese, Korean, Malay, Romanian, Russian, Spanish, and Turkish. In some later editions, the eighteenth chapter, titled ''Nanostructures'', was written by Paul McEuen. Along with its competitor '' Ashcroft and Mermin'', the book is considered a standard textbook in condensed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thin-film Solar Cells
Thin-film solar cells are a type of solar cell made by depositing one or more thin layers (thin films or TFs) of photovoltaic material onto a substrate, such as glass, plastic or metal. Thin-film solar cells are typically a few nanometers ( nm) to a few microns ( μm) thick–much thinner than the wafers used in conventional crystalline silicon (c-Si) based solar cells, which can be up to 200 μm thick. Thin-film solar cells are commercially used in several technologies, including cadmium telluride (CdTe), copper indium gallium diselenide (CIGS), and amorphous thin-film silicon (a-Si, TF-Si). Solar cells are often classified into so-called generations based on the active (sunlight-absorbing) layers used to produce them, with the most well-established or ''first-generation'' solar cells being made of single- or multi-crystalline silicon. This is the dominant technology currently used in most solar PV systems. Most thin-film solar cells are classified as ''second gene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycrystalline
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel longulites. Structure The orientation of crystallites can be random with no preferred direction, called random texture, or directed, possibly due to growth and processing conditions. While the structure of a single crystal is highly ordered and its lattice is continuous and unbroken, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are polycrystalline, made of a large number crystallites held together by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GaAs
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure. Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated circuits, infrared light-emitting diodes, laser diodes, solar cells and optical windows. GaAs is often used as a substrate material for the epitaxial growth of other III-V semiconductors, including indium gallium arsenide, aluminum gallium arsenide and others. History Gallium arsenide was first synthesized and studied by Victor Goldschmidt in 1926 by passing arsenic vapors mixed with hydrogen over gallium(III) oxide at 600 °C. The semiconductor properties of GaAs and other III-V compounds were patented by Heinrich Welker at Siemens-Schuckert in 1951 and described in a 1952 publication. Commercial production of its monocrystals commenced in 1954, and more studies followed in the 1950s. First infrared LEDs were made in 1962. Preparatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Selenide
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. (Note that incorrectly identifies PbSe and other IV–VI semiconductors as indirect gap materials.) A grey solid, it is used for manufacture of infrared detectors for thermal imaging. The mineral clausthalite is a naturally occurring lead selenide. It may be formed by direct reaction between its constituent elements, lead and selenium. Infrared detection PbSe was one of the first materials found to be sensitive to the infrared radiation used for military applications. Early research works on the material as infrared detector were carried out during the 1930s and the first useful devices were processed by Germans, Americans and British during and just after World War II. Since then, PbSe has been commonly used as an infrared photodetector in multiple applications, from spectro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Arsenide
Indium arsenide, InAs, or indium monoarsenide, is a narrow-bandgap semiconductor composed of indium and arsenic. It has the appearance of grey cubic crystals with a melting point of 942 °C. Indium arsenide is similar in properties to gallium arsenide and is a direct bandgap material, with a bandgap of 0.35 eV at room temperature. Indium arsenide is used for the construction of infrared detectors, for the wavelength range of 1.0–3.8 μm. The detectors are usually photovoltaic photodiodes. Cryogenically cooled detectors have lower noise, but InAs detectors can be used in higher-power applications at room temperature as well. Indium arsenide is also used for making diode lasers. InAs is well known for its high electron mobility and narrow energy bandgap. It is widely used as a terahertz radiation Terahertz radiation – also known as submillimeter radiation, terahertz waves, tremendously high frequency (THF), T-rays, T-waves, T-light, T-lux or THz – consis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Antimonide
Gallium antimonide (GaSb) is a semiconducting compound of gallium and antimony of the III-V family. It has a room temperature lattice constant of about 0.610 nm. It has a room temperature direct bandgap of approximately 0.73 eV. History The intermetallic compound GaSb was first prepared in 1926 by Victor Goldschmidt, who directly combined the elements under an inert gas atmosphere and reported on GaSb's lattice constant, which has since been revised. Goldschmidt also synthesized gallium phosphide and gallium arsenide. The Ga-Sb phase equilibria was investigated in 1955 by Koster and by Greenfield. Applications GaSb can be used for Infrared detectors, infrared LEDs and lasers and transistors, and thermophotovoltaic systems. See also * Aluminium antimonide * Indium antimonide * Gallium arsenide Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a Zincblende (crystal structure), zinc blende crystal structure. Gallium arsenide is used in the manufacture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Antimonide
Aluminium (or aluminum in North American English) is a chemical element; it has symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has a great affinity towards oxygen, forming a protective layer of oxide on the surface when exposed to air. It visually resembles silver, both in its color and in its great ability to reflect light. It is soft, nonmagnetic, and ductile. It has one stable isotope, 27Al, which is highly abundant, making aluminium the 12th-most abundant element in the universe. The radioactivity of 26Al leads to it being used in radiometric dating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it has more polarizing power, and bonds formed by aluminium have a more covalent character. The stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lattice Constant
A lattice constant or lattice parameter is one of the physical dimensions and angles that determine the geometry of the unit cells in a crystal lattice, and is proportional to the distance between atoms in the crystal. A simple cubic crystal has only one lattice constant, the distance between atoms, but, in general, lattices in three dimensions have six lattice constants: the lengths ''a'', ''b'', and ''c'' of the three cell edges meeting at a vertex, and the angles ''α'', ''β'', and ''γ'' between those edges. The crystal lattice parameters ''a'', ''b'', and ''c'' have the dimension of length. The three numbers represent the size of the unit cell, that is, the distance from a given atom to an identical atom in the same position and orientation in a neighboring cell (except for very simple crystal structures, this will not necessarily be distance to the nearest neighbor). Their SI unit is the meter, and they are traditionally specified in angstroms (Å); an angstrom being 0.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |