|
Wet Oxidation
Wet oxidation is a form of hydrothermal treatment. It is the oxidation of dissolved or suspended components in water using oxygen as the oxidizer. It is referred to as wet air oxidation (WAO) when air is used. The oxidation reactions occur in superheated water at a temperature above the normal boiling point of water (100 °C), but below the critical point (374 °C). The system must be maintained under pressure to avoid excessive evaporation of water. This is done to control energy consumption due to the latent heat of vaporization. It is also done because liquid water is necessary for most of the oxidation reactions to occur. Compounds oxidize under wet oxidation conditions that would not oxidize under dry conditions at the same temperature and pressure. Wet oxidation has been used commercially for around 60 years. It is used predominantly for treating wastewater. It is often referred to as Zimpro (from ZIMmerman PROcess), after Fred J. Zimmermann who commercial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spent Caustic
Spent caustic is a waste industrial caustic solution that has become exhausted and is no longer useful (or spent). Spent caustics are made of sodium hydroxide or potassium hydroxide, water, and contaminants. The contaminants have consumed the majority of the sodium (or potassium) hydroxide and thus the caustic liquor is spent, for example, in one common application H2S (''gas'') is scrubbed by the NaOH (''aqueous'') to form NaHS (''aq'') and H2O (''l''), thus consuming the caustic. Types * Ethylene spent caustic comes from the caustic scrubbing of cracked gas from an ethylene cracker. This liquor is produced by a caustic scrubbing tower. Ethylene product gas is contaminated with (g) and (g), and those contaminants are removed by absorption in the caustic scrubbing tower to produce (aq) and {{chem, Na, 2, CO, 3(aq). The sodium hydroxide is consumed and the resulting wastewater (ethylene spent caustic) is contaminated with the sulfides and carbonates and a small fraction of organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Treatment
Thermal treatment is any list of solid waste treatment technologies, waste treatment technology that involves high temperatures in the processing of the waste Raw material, feedstock. Commonly this involves the combustion of waste materials. Systems that are generally considered to be thermal treatment include: *Cement kiln *Gasification *Incineration *Mechanical heat treatment *Pyrolysis *Thermal depolymerization *Waste autoclaves See also *Anaerobic digestion *List of solid waste treatment technologies *Mechanical biological treatment *Waste-to-energy *Pyrolysis References {{Waste Thermal treatment, Waste management Waste treatment technology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Processes
A chemical substance is a unique form of matter with constant chemical composition and characteristic properties. Chemical substances may take the form of a single element or chemical compounds. If two or more chemical substances can be combined without reacting, they may form a chemical mixture. If a mixture is separated to isolate one chemical substance to a desired degree, the resulting substance is said to be chemically pure. Chemical substances can exist in several different physical states or phases (e.g. solids, liquids, gases, or plasma) without changing their chemical composition. Substances transition between these phases of matter in response to changes in temperature or pressure. Some chemical substances can be combined or converted into new substances by means of chemical reactions. Chemicals that do not possess this ability are said to be inert. Pure water is an example of a chemical substance, with a constant composition of two hydrogen atoms bonded to a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Waste-water Treatment Technologies
This page consists of a list of wastewater treatment technologies: See also *Agricultural wastewater treatment *Industrial wastewater treatment *List of solid waste treatment technologies * Waste treatment technologies *Water purification Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for hu ... * Sewage sludge treatment References * * Industrial Wastewater Treatment Technology DatabaseEPA. {{DEFAULTSORT:List Of Waste Water Treatment Technologies Chemical processes Environmental engineering *List Water pollution Water technology Waste-water treatment technologies Sanitation * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incineration
Incineration is a waste treatment process that involves the combustion of substances contained in waste materials. Industrial plants for waste incineration are commonly referred to as waste-to-energy facilities. Incineration and other high-temperature waste treatment systems are described as "thermal treatment". Incineration of waste materials converts the waste into ash, flue gas and heat. The ash is mostly formed by the inorganic constituents of the waste and may take the form of solid lumps or particulates carried by the flue gas. The flue gases must be cleaned of gaseous and particulate pollutants before they are dispersed into the atmosphere. In some cases, the heat that is generated by incineration can be used to generate electric power. Incineration with energy recovery is one of several waste-to-energy technologies such as gasification, pyrolysis and anaerobic digestion. While incineration and gasification technologies are similar in principle, the energy produce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
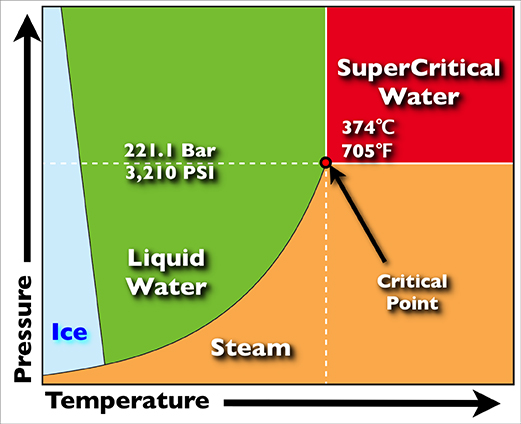
Supercritical Water Oxidation
Supercritical water oxidation (SCWO) is a process that occurs in water at temperatures and pressures above a mixture's thermodynamic critical point. Under these conditions water becomes a fluid with unique properties that can be used to advantage in the destruction of recalcitrant and hazardous wastes such as polychlorinated biphenyls (PCB) or per- and polyfluoroalkyl substances (PFAS). Supercritical water has a density between that of water vapor and liquid at standard conditions, and exhibits high gas-like diffusion rates along with high liquid-like collision rates. In addition, the behavior of water as a solvent is altered (in comparison to that of subcritical liquid water) - it behaves much less like a polar solvent. As a result, the solubility behavior is "reversed" so that oxygen, and organics such as chlorinated hydrocarbons become soluble in the water, allowing single-phase reaction of aqueous waste with a dissolved oxidizer. The reversed solubility also causes salts to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Powdered Activated Carbon Treatment
Powdered Activated Carbon Treatment (PACT) is a wastewater technology in which powdered activated carbon is added to an anaerobic or aerobic treatment system.Jafarinejad, S. “Activated sludge combined with powdered activated carbon (PACT process) for the petroleum industry wastewater treatment: A review.” Chemistry International. 3(4) (2017) 26. The carbon in the biological treatment process adsorbs recalcitrant compounds that are not readily biodegradable, thereby reducing the chemical oxygen demand of the wastewater and removing toxins.Meidl, John. “Use of the PACT System to Treat Industrial Wastewaters for Direct Discharge or Reuse.” Jubilee XX Conference of Science & Technology. The carbon also acts as a "buffer" against the effects of toxic organics in the wastewater. In such a system, biological treatment and carbon adsorption are combined into a single, synergistic treatment step. The result is a system which offers significant cost reduction compared to acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mother Liquor
The mother liquor (or spent liquor) is the Solution (chemistry), solution remaining after a component has been removed by a process such as filtration or more commonly crystallization. It is encountered in chemical processes including sugar refining. In crystallization, a solid (usually impure) is dissolved in a solvent at high temperature, taking advantage of the fact that most solids are more soluble at higher temperatures. As the solution cools, the solubility of the solute in the solvent will gradually become smaller. The resultant solution is described as supersaturation, supersaturated, meaning that there is more solute dissolved in the solution than would be predicted by its solubility at that temperature. Crystallization can then be induced from this supersaturated solution and the resultant pure crystals removed by such methods as filtration and centrifugal separators. The remaining solution, once the crystals have been filtered out, is known as the mother liquor, and will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers, and is typically made from benzene for this purpose. Approximately 25 million tonnes of styrene were produced in 2010, increasing to around 35 million tonnes by 2018. Natural occurrence Styrene is named after storax balsam (often commercially sold as ''styrax''), the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, balsam tree (other), balsam trees and peanuts) and is also found in coal tar. History In 1839, the German apothecary Eduard Simon isolated a volatile liquid from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pasteurize
In food processing, pasteurization ( also pasteurisation) is a process of food preservation in which packaged foods (e.g., milk and fruit juices) are treated with mild heat, usually to less than , to eliminate pathogens and extend shelf life. Pasteurization either destroys or deactivates microorganisms and enzymes that contribute to food spoilage or the risk of disease, including vegetative bacteria, but most bacterial spores survive the process. Pasteurization is named after the French microbiologist Louis Pasteur, whose research in the 1860s demonstrated that thermal processing would deactivate unwanted microorganisms in wine. Spoilage enzymes are also inactivated during pasteurization. Today, pasteurization is used widely in the dairy industry and other food processing industries for food preservation and food safety. By the year 1999, most liquid products were heat treated in a continuous system where heat was applied using a heat exchanger or the direct or indirect use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |