|
WR 140
WR 140 is a visually moderately bright Wolf–Rayet star placed within the spectroscopic binary star, SBC9 1232, whose primary star is an Stellar evolution, evolved spectral O-type star, class O4–5 star. It is located in the constellation of Cygnus (constellation), Cygnus, lying in the sky at the centre of the triangle formed by Deneb, Gamma Cygni, γ Cygni and Delta Cygni, δ Cygni. Significance WR 140 is thought to be a prototypical example of cosmic dust production. In this mode of cosmic dust production, detritus enriched in silicon and carbon is periodically blown into the wider universe by certain stars toward the end of their lives. Such stars are termed Wolf–Rayets. The outermost layers of a Wolf–Rayet star are Nucleosynthesis, enriched in oxygen, nitrogen, silicon and carbon. Indeed, the spectrographic presence of these elements, along with a notable absence of hydrogen, were one of the original diagnostic criteria for classifying a star as Wolf–Ray ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deneb
Deneb () is a blue supergiant star in the constellation of Cygnus. It is the brightest star in the constellation and the 19th brightest in the night sky, with an apparent magnitude slightly varying between +1.21 and +1.29. Deneb is one of the vertices of the asterism known as the Summer Triangle and the "head" of the Northern Cross. Its Bayer designation is α Cygni, which is Latinised to Alpha Cygni, abbreviated to Alpha Cyg or α Cyg. Deneb rivals Rigel, a closer blue supergiant, as the most luminous first-magnitude star. However, its distance, and hence luminosity, is poorly known; its luminosity is estimated to be between 55,000 and 196,000 times that of the Sun. Distance estimates range from 1,400 to 2,600 light-years; assuming its highest value, it is the farthest star with an apparent magnitude brighter than 2.50. Nomenclature ''α Cygni'' (Latinised to ''Alpha Cygni'') is the star's designation given by Johann Bayer in 1603. The traditional name ''De ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metallicity
In astronomy, metallicity is the Abundance of the chemical elements, abundance of Chemical element, elements present in an object that are heavier than hydrogen and helium. Most of the normal currently detectable (i.e. non-Dark matter, dark) matter in the universe is either hydrogen or helium, and astronomers use the word ''metals'' as convenient shorthand for ''all elements except hydrogen and helium''. This word-use is distinct from the conventional chemical or physical definition of a metal as an electrically conducting element. Stars and nebulae with relatively high abundances of heavier elements are called ''metal-rich'' when discussing metallicity, even though many of those elements are called ''Nonmetal (chemistry), nonmetals'' in chemistry. Metals in early spectroscopy In 1802, William Hyde WollastonMelvyn C. UsselmanWilliam Hyde WollastonEncyclopædia Britannica, retrieved 31 March 2013 noted the appearance of a number of dark features in the solar spectrum. In 1814, Jo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Webb Space Telescope
The James Webb Space Telescope (JWST) is a space telescope designed to conduct infrared astronomy. As the largest telescope in space, it is equipped with high-resolution and high-sensitivity instruments, allowing it to view objects too old, List of the most distant astronomical objects, distant, or faint for the Hubble Space Telescope. This enables investigations across many fields of astronomy and cosmology, such as observation of the Population III star, first stars and the Galaxy formation and evolution, formation of the first galaxies, and detailed atmospheric characterization of potentially habitable exoplanets. Although the Webb's mirror diameter is 2.7 times larger than that of the Hubble Space Telescope, it produces images of comparable optical resolution, resolution because it observes in the longer-wavelength infrared spectrum. The longer the wavelength of the spectrum, the larger the information-gathering surface required (mirrors in the infrared spectrum or antenna a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Astronomy
Infrared astronomy is a sub-discipline of astronomy which specializes in the astronomical observation, observation and analysis of astronomical objects using infrared (IR) radiation. The wavelength of infrared light ranges from 0.75 to 300 micrometers, and falls in between Visible light, visible radiation, which ranges from 380 to 750 nanometers, and terahertz radiation, submillimeter waves. Infrared astronomy began in the 1830s, a few decades after the discovery of infrared light by William Herschel in 1800. Early progress was limited, and it was not until the early 20th century that conclusive detections of astronomical objects other than the Sun and Moon were made in infrared light. After a number of discoveries were made in the 1950s and 1960s in radio astronomy, astronomers realized the information available outside the visible wavelength range, and modern infrared astronomy was established. Infrared and optical astronomy are often practiced using the same telescopes, as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By definition, the Celsius scale (symbol °C) and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15. The 19th century British scientist Lord Kelvin first developed and proposed the scale. It was often called the "absolute Celsius" scale in the early 20th century. The kelvin was formally added to the International System of Units in 1954, defining 273.16 K to be the triple point of water. The Celsius, Fahrenheit, and Rankine scales were redefined in terms of the Kelvin scale using this definition. The 2019 revision of the SI now defines the kelvin in terms of energy by setting the Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Silicon is a significant element that is essential for several physiological and metabolic processes in plants. Silicon is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others. These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
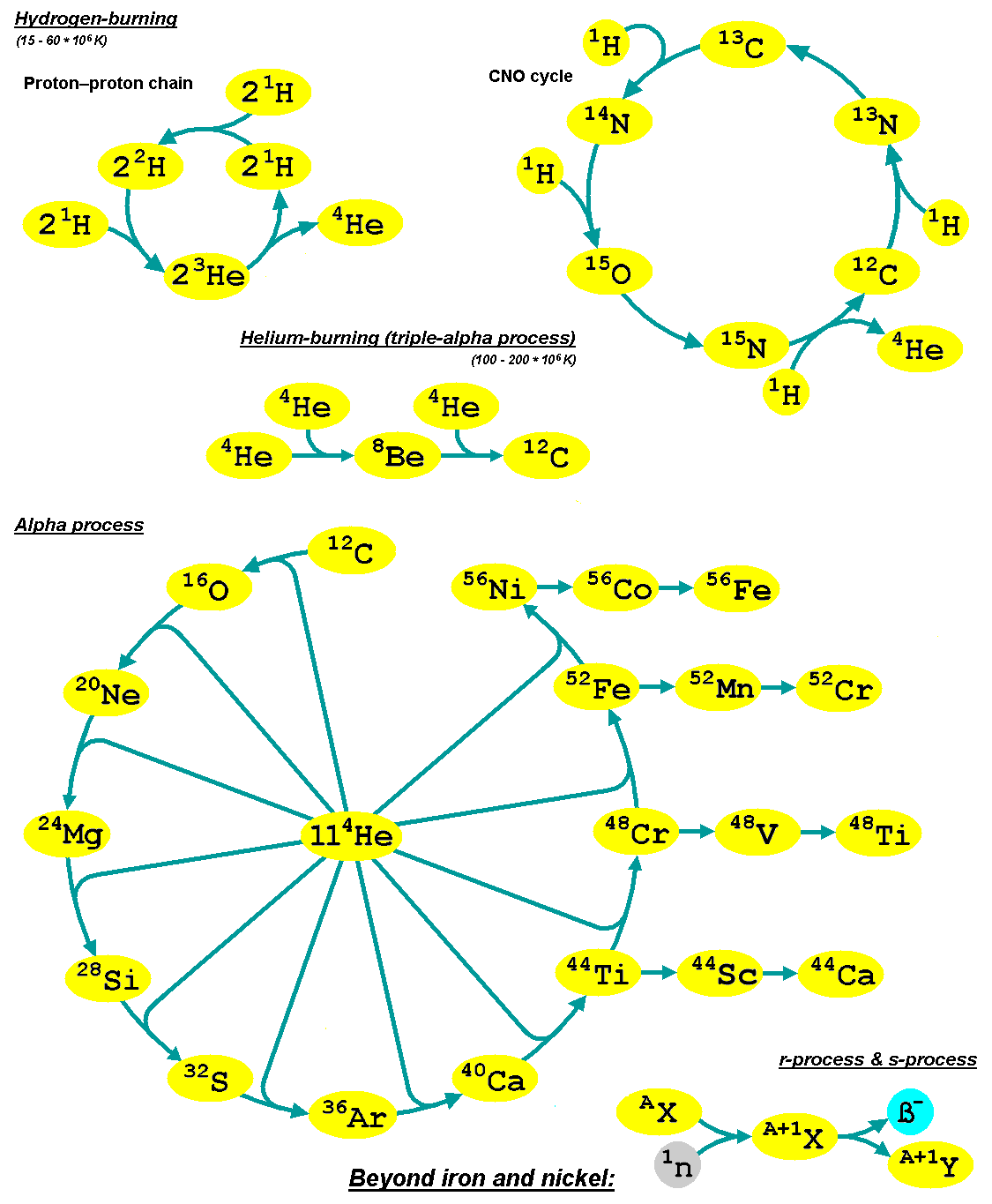
Nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons (protons and neutrons) and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing hydrogen and helium. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium (called 'metals' by astrophysicists) remains small (few percent), so that the universe still has approximately the same composition. Stars ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |