|
WIPI Protein Family
The WIPI protein family (WD-repeat protein Interacting with PhosphoInositides) is an evolutionarily conserved family of proteins. WIPI proteins contain a WD repeat domain that folds into a 7-bladed beta-propeller that functions in autophagy Autophagy (or autophagocytosis; from the Ancient Greek , , meaning "self-devouring" and , , meaning "hollow") is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent re ..., and contain a conserved motif for interaction with phospholipids. Members of this family include: References Protein families {{Protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WD Repeat
The WD40 repeat (also known as the WD or beta-transducin repeat) is a short structural motif of approximately 40 amino acids, often terminating in a tryptophan-aspartic acid (W-D) dipeptide. Tandem copies of these repeats typically fold together to form a type of circular solenoid protein domain called the WD40 domain. Structure WD40 domain-containing proteins have 4 to 16 repeating units, all of which are thought to form a circularised beta-propeller structure (see figure to the right). The WD40 domain is composed of several repeats, a variable region of around 20 residues at the beginning followed by a more common repeated set of residues. These repeats typically form a four stranded anti-parallel beta sheet or blade. These blades come together to form a propeller with the most common being a 7 bladed beta propeller. The blades interlock so that the last beta strand of one repeat forms with the first three of the next repeat to form the 3D blade structure. Function WD40-repe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-propeller
In structural biology, a beta-propeller (β-propeller) is a type of all-β protein architecture characterized by 4 to 8 highly symmetrical blade-shaped beta sheets arranged toroidally around a central axis. Together the beta-sheets form a funnel-like active site. Structure Each beta-sheet typically has four anti-parallel β-strands arranged in the beta-zigzag motif. The strands are twisted so that the first and fourth strands are almost perpendicular to each other. There are five classes of beta-propellers, each arrangement being a highly symmetrical structure with 4–8 beta sheets, all of which generally form a central tunnel that yields pseudo-symmetric axes. While, the protein's official active site for ligand-binding is formed at one end of the central tunnel by loops between individual beta-strands, protein-protein interactions can occur at multiple areas around the domain. Depending on the packing and tilt of the beta-sheets and beta-strands, the beta-propeller may ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
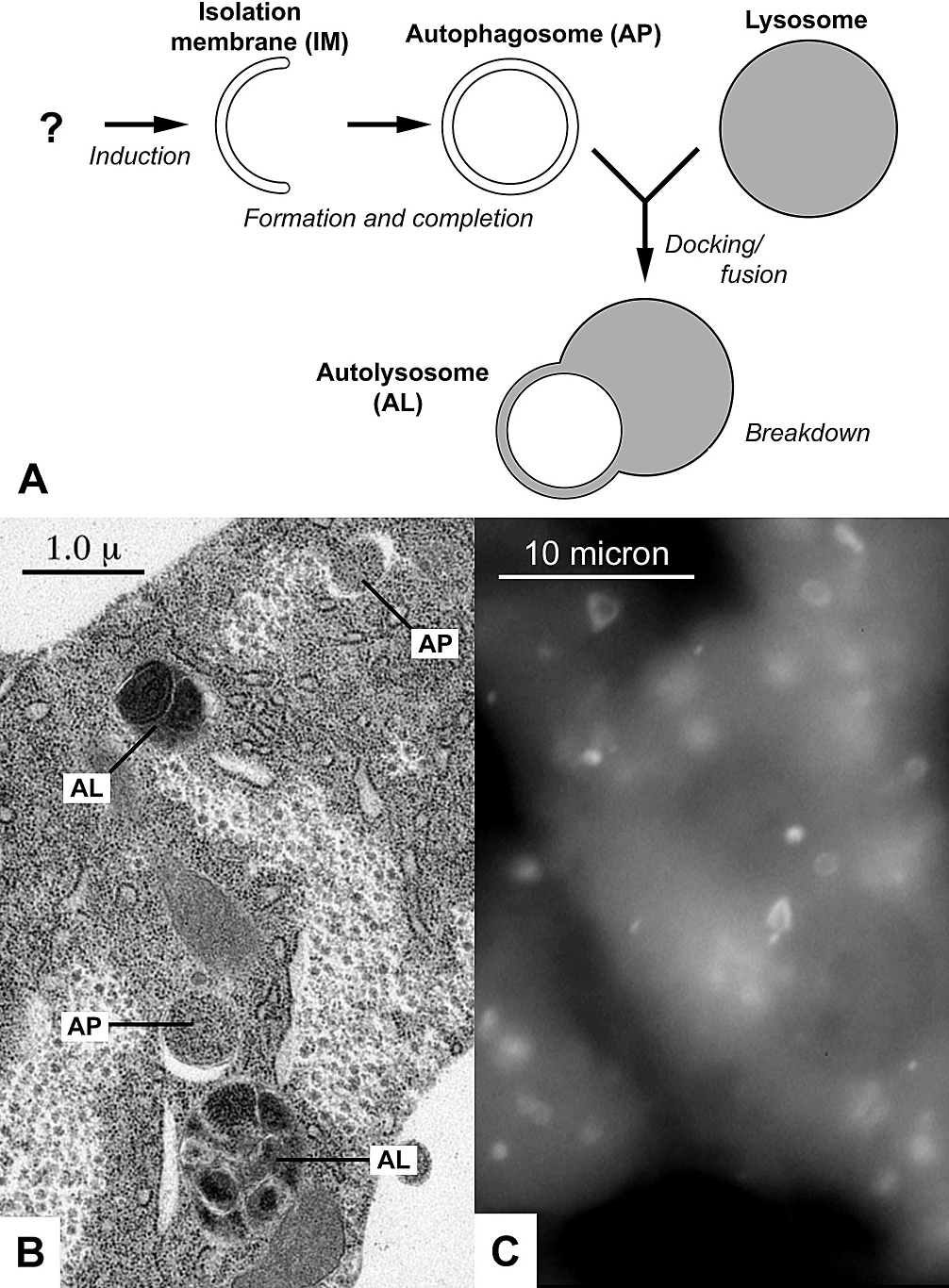
Autophagy
Autophagy (or autophagocytosis; from the Ancient Greek , , meaning "self-devouring" and , , meaning "hollow") is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent regulated mechanism. It allows the orderly degradation and recycling of cellular components. Although initially characterized as a primordial degradation pathway induced to protect against starvation, it has become increasingly clear that autophagy also plays a major role in the homeostasis of non-starved cells. Defects in autophagy have been linked to various human diseases, including neurodegeneration and cancer, and interest in modulating autophagy as a potential treatment for these diseases has grown rapidly. Four forms of autophagy have been identified: macroautophagy, microautophagy, chaperone-mediated autophagy (CMA), and crinophagy. In macroautophagy (the most thoroughly researched form of autophagy), cytoplasmic components (like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WIPI1
WD repeat domain phosphoinositide-interacting protein 1 (WIPI-1), also known as Atg18 protein homolog (ATG18) and WD40 repeat protein interacting with phosphoinositides of 49 kDa (WIPI 49 kDa), is a protein that in humans is encoded by the ''WIPI1'' gene. Structure and function WD40 repeat proteins are key components of many essential biologic functions. They regulate the assembly of multiprotein complexes by presenting a beta-propeller platform for simultaneous and reversible protein–protein interactions. Members of the WIPI subfamily of WD40 repeat proteins, such as WIPI1, have a 7-bladed propeller structure and contain a conserved motif for interaction with phospholipid Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...s. See also * WIPI protein family References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WIPI2
WD repeat domain phosphoinositide-interacting protein 2 is a protein that in humans is encoded by the ''WIPI2'' gene. Function WD40 repeat proteins are key components of many essential biologic functions. They regulate the assembly of multiprotein complexes by presenting a beta-propeller platform for simultaneous and reversible protein-protein interactions. Members of the WIPI subfamily of WD40 repeat proteins, such as WIPI2, have a 7-bladed propeller structure and contain a conserved motif for interaction with phospholipids. WIPI2 is the mammalian homolog of Atg18, not Atg21, along with the closely related protein, WIPI1. WIPI2 mRNA is readily detectable in several commonly used laboratory cell lines (HEK293A, HeLa, A431) and several cancer cell lines, while WIPI1 expression is limited to cancer cells (but is also detected in many human tissues). The Atg proteins regulate autophagy Autophagy (or autophagocytosis; from the Ancient Greek , , meaning "self-devouring" an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WDR45L
WD repeat domain phosphoinositide-interacting protein 3 (WIPI-3), also known as WD repeat-containing protein 45-like is a protein that in humans is encoded by the gene. Structure and function WIPI-3 is a member of the WIPI or SVP1 family of WD40 repeat-containing proteins. The protein contains seven WD40 repeats that are thought to fold into a beta-propeller structure that mediates protein–protein interactions, and a conserved motif for interaction with phospholipids. See also * WIPI protein family The WIPI protein family (WD-repeat protein Interacting with PhosphoInositides) is an evolutionarily conserved family of proteins. WIPI proteins contain a WD repeat domain that folds into a 7-bladed beta-propeller In structural biology, a beta-pro ... References Further reading * * * {{gene-17-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WDR45
WD repeat domain phosphoinositide-interacting protein 4 (WIPI-4) is a protein that in humans is encoded by the ''WDR45'' gene. Mutations in this gene cause a distinct form of ''Neurodegeneration with brain iron accumulation'' (NBIA) called Beta-propeller protein-associated neurodegeneration (BPAN). Function WIPI-4 is a member of the WD repeat protein family. WD repeats are minimally conserved regions of approximately 40 amino acids typically bracketed by gly-his and trp-asp (GH-WD), which may facilitate formation of heterotrimeric or multiprotein complexes. Members of this family are involved in a variety of cellular processes, including cell cycle progression, signal transduction Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellular ..., apoptosis, and gene regulation. This gene WDR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |