|
Vpu
Vpu is an accessory protein that in HIV is encoded by the ''vpu'' gene. Vpu stands for "Viral Protein U". The Vpu protein acts in the degradation of CD4 in the endoplasmic reticulum and in the enhancement of virion release from the plasma membrane of infected cells. Vpu induces the degradation of the CD4 viral receptor and therefore participates in the general downregulation of CD4 expression during the course of HIV infection. Vpu-mediated CD4 degradation is thought to prevent CD4-Env binding in the endoplasmic reticulum to facilitate proper Env assembly into virions. It is found in the membranes of infected cells, but not the virus particles themselves. The Vpu gene is found exclusively in HIV-1 and some HIV-1-related simian immunodeficiency virus ( SIV) isolates, such as SIV, SIV, and SIV, but not in HIV-2 or the majority of SIV isolates. Structural similarities between Vpu and another small viral protein, M2, encoded by influenza A virus were first noted soon after the discove ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Simian Immunodeficiency Virus
Simian immunodeficiency virus (SIV) is a species of retrovirus that cause persistent infections in at least 45 species of non-human primates. Based on analysis of strains found in four species of monkeys from Bioko Island, which was isolated from the mainland by rising sea levels about 11,000 years ago, it has been concluded that SIV has been present in monkeys and apes for at least 32,000 years, and probably much longer. Virus strains from three of these primate species, SIVsmm in sooty mangabeys, SIVgor in gorillas and SIVcpz in chimpanzees, are believed to have crossed the species barrier into humans, resulting in HIV-2 and HIV-1 respectively, the two HIV viruses. The most likely route of transmission of HIV-1 to humans involves contact with the blood of chimps and gorillas that are often hunted for bushmeat in Africa. Four subtypes of HIV-1 (M, N, O, and P) likely arose through four separate transmissions of SIV to humans, and the resulting HIV-1 group M strain most ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BST2
Tetherin, also known as bone marrow stromal antigen 2, is a lipid raft associated protein that in humans is encoded by the ''BST2'' gene. In addition, tetherin has been designated as CD317 (cluster of differentiation 317). This protein is constitutively expressed in mature B cells, plasma cells and plasmacytoid dendritic cells, and in many other cells, it is only expressed as a response to stimuli from IFN pathway. Gene activation Tetherin is part of IFN-dependent antiviral response pathway. When the presence of virus and viral components is detected by recognition molecules such as (RIG-I), a cascades of interactions happen between signaling molecules, eventually the signal reaches the nucleus to upregulate the expression of interferon-stimulated genes (ISGs), this in turn activates IFN-α pathway to send the signal to neighboring cells, which causes upregulation in the expression of other ISGs and many viral restriction factors, such as tetherin. Tetherin/BST2 and BST1 g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accessory Protein
A viral regulatory and accessory protein is a type of viral protein that can play an indirect role in the function of a virus. An example is Nef (protein), Nef. References Further reading * Viral proteins {{virus-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoplasmic
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are the cytosol (a gel-like substance), the cell's internal sub-structures, and various cytoplasmic inclusions. In eukaryotes the cytoplasm also includes the nucleus, and other membrane-bound organelles.The cytoplasm is about 80% water and is usually colorless. The submicroscopic ground cell substance, or cytoplasmic matrix, that remains after the exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all resolvable cytoplasmic elements are suspended, including the larger organelles such as the ribosomes, mitochondria, plant plastids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Casein Kinase 2
Casein kinase 2 ()(CK2/CSNK2) is a serine/threonine-selective protein kinase that has been implicated in cell cycle control, DNA repair, regulation of the circadian rhythm, and other cellular processes. De-regulation of CK2 has been linked to tumorigenesis as a potential protection mechanism for mutated cells. Proper CK2 function is necessary for survival of cells as no knockout models have been successfully generated. Structure CK2 typically appears as a tetramer of two α subunits; α being 42 kDa and α’ being 38 kDa, and two β subunits, each weighing in at 28 kDa. The β regulatory domain only has one isoform and therefore within the tetramer will have two β subunits. The catalytic α domains appear as an α or α’ variant and can either be formed in a homodimer (α & α, or α’ & α’) formation or heterodimer formation (α & α’). Other β isoforms have been found in other organisms but not in humans. The α subunits do not require the β regulatory subunits to f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
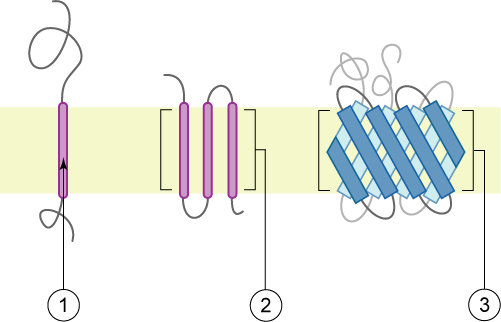
Type I Membrane Protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to the network of interactions between different proteins in cells, including interactions via transmembrane alpha helices. They usually include one or several water-soluble domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. More than 2300 single-pass membrane proteins were identified in the human genome. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligomeric Protein
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multidomain enzymes, in which multiple catalytic domains are found in a single polypeptide chain. Protein complexes are a form of quaternary structure. Proteins in a protein complex are linked by non-covalent protein–protein interactions. These complexes are a cornerstone of many (if not most) biological processes. The cell is seen to be composed of modular supramolecular complexes, each of which performs an independent, discrete biological function. Through proximity, the speed and selectivity of binding interactions between enzymatic complex and substrates can be vastly improved, leading to higher cellular efficiency. Many of the techniques used to enter cells and isolate proteins are inherently disruptive to such large complexes, complicating the task of determining the components of a complex. Examples of protein complexes include the pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Raft
The cell membrane, plasma membranes of cells contain combinations of glycosphingolipids, cholesterol and protein Receptor (biochemistry), receptors organized in glycolipoprotein lipid microdomains termed lipid rafts. Their existence in cellular membranes remains controversial. Indeed, Kervin and Overduin imply that lipid rafts are misconstrued protein islands, which they propose form through a proteolipid code. Nonetheless, it has been proposed that they are specialized membrane microdomains which compartmentalize cellular processes by serving as organising centers for the assembly of signaling molecules, allowing a closer interaction of protein receptors and their effectors to promote kinetically favorable interactions necessary for the signal transduction. Lipid rafts influence membrane fluidity and membrane protein Protein targeting, trafficking, thereby regulating neurotransmission and receptor trafficking. Lipid rafts are more ordered and tightly packed than the surrounding bil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylphosphatidylinositol
Glycosylphosphatidylinositol () or glycophosphatidylinositol (GPI) is a phosphoglyceride that can be attached to the C-terminus of a protein during posttranslational modification. The resulting GPI-anchored proteins play key roles in a wide variety of biological processes. GPI is composed of a phosphatidylinositol group linked through a carbohydrate-containing linker (glucosamine and mannose glycosidically bound to the inositol residue) and via an ethanolamine phosphate (EtNP) bridge to the C-terminal amino acid of a mature protein. The two fatty acids within the hydrophobic phosphatidyl-inositol group anchor the protein to the cell membrane. Synthesis Glycosylated (GPI-anchored) proteins contain a signal sequence, thus directing them to the endoplasmic reticulum (ER). The protein is co-translationally inserted in the ER membrane via a translocon and is attached to the ER membrane by its hydrophobic C terminus; the majority of the protein extends into the ER lumen. The hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |