|
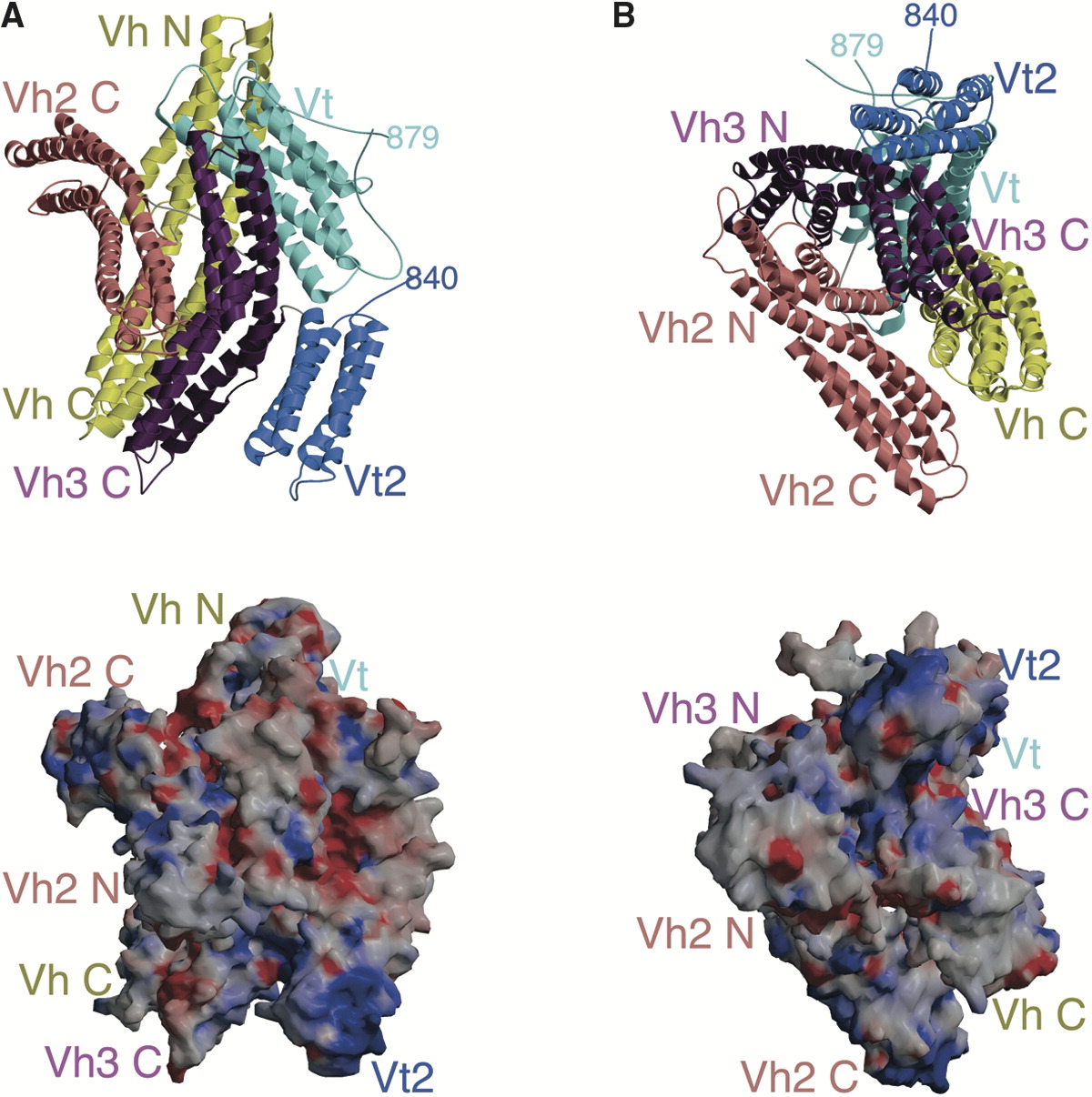
Vinculin Family
Vinculin is a eukaryotic protein that seems to be involved in the attachment of the actin-based microfilaments to the plasma membrane. Vinculin is located at the cytoplasmic side of focal adhesion, focal contacts or adhesion plaques. In addition to actin, vinculin interacts with other structural proteins such as talin protein, talin and alpha-actinins. Vinculin is a large protein of 116 kDa (about a 1000 residues). Structurally the protein consists of an acidic N-terminal domain of about 90 kDa separated from a basic C-terminal domain of about 25 kDa by a proline-rich region of about 50 residues. The central part of the N-terminal domain consists of a variable number (3 in vertebrates, 2 in ''Caenorhabditis elegans'') of repeats of a 110 amino acids domain. Alpha-catenins are evolutionary related to vinculin. Catenins are proteins that associate with the cytoplasmic domain of a variety of cadherins. The association of catenins to cadherins produces a complex which is linked ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinculin Domain Structure
In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane. Discovered independently by Benny Geiger and Keith Burridge, its sequence is 20%–30% similar to α-catenin, which serves a similar function. Binding alternately to talin or α-actinin, vinculin's shape and, as a consequence, its binding properties are changed. The vinculin gene occurs as a single copy and what appears to be no close relative to take over functions in its absence. Its splice variant metavinculin (see below) also needs vinculin to heterodimerize and work in a dependent fashion. Structure Vinculin is a 117-kDa cytoskeletal protein with 1066 amino acids. The protein contains an acidic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CTNNAL1
Alpha-catulin is a protein that in humans is encoded by the ''CTNNAL1'' gene. Interactions CTNNAL1 has been shown to Protein-protein interaction, interact with AKAP13. References External links * Further reading * * * * * * * * {{gene-9-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CTNNA1
αE-catenin, also known as Catenin alpha-1 is a protein that in humans is encoded by the ''CTNNA1'' gene. αE-catenin is highly expressed in cardiac muscle and localizes to adherens junctions at intercalated disc structures where it functions to mediate the anchorage of actin filaments to the sarcolemma. αE-catenin also plays a role in tumor metastasis and skin cell function. Structure Human αE-catenin protein is 100.0 kDa and 906 amino acids. Catenins (α,β,and γ (also known as plakoglobin)) were originally identified in complex with E-cadherin, an epithelial cell adhesion protein. αE-catenin is highly expressed in cardiac muscle and is homologous to the protein vinculin; however, aside from vinculin, αE-catenin has no homology to established actin-binding proteins. The N-terminus of αE-catenin binds β-catenin or γ-catenin/plakoglobin, and the C-terminus binds actin directly or indirectly via vinculin or α-actinin. Function Though αE-catenin exhibits substantial exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadherin
Cadherins (named for "calcium-dependent adhesion") are cell adhesion molecules important in forming adherens junctions that let cells adhere to each other. Cadherins are a class of type-1 transmembrane proteins, and they depend on calcium (Ca2+) ions to function, hence their name. Cell-cell adhesion is mediated by extracellular cadherin domains, whereas the Cadherin cytoplasmic region, intracellular cytoplasmic tail associates with numerous adaptors and signaling proteins, collectively referred to as the cadherin adhesome. Background The cadherin family is essential in maintaining cell-cell contact and regulating cytoskeletal complexes. The cadherin superfamily includes cadherins, protocadherins, desmogleins, desmocollins, and more. In structure, they share ''cadherin repeats'', which are the extracellular Ca2+-binding domains. There are multiple classes of cadherin molecules, each designated with a prefix for tissues with which it associates. Classical cadherins maintain the ton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caenorhabditis Elegans
''Caenorhabditis elegans'' () is a free-living transparent nematode about 1 mm in length that lives in temperate soil environments. It is the type species of its genus. The name is a Hybrid word, blend of the Greek ''caeno-'' (recent), ''rhabditis'' (rod-like) and Latin ''elegans'' (elegant). In 1900, Émile Maupas, Maupas initially named it ''Rhabditidae, Rhabditides elegans.'' Günther Osche, Osche placed it in the subgenus ''Caenorhabditis'' in 1952, and in 1955, Ellsworth Dougherty, Dougherty raised ''Caenorhabditis'' to the status of genus. ''C. elegans'' is an unsegmented pseudocoelomate and lacks respiratory or circulatory systems. Most of these nematodes are hermaphrodites and a few are males. Males have specialised tails for mating that include spicule (nematode), spicules. In 1963, Sydney Brenner proposed research into ''C. elegans,'' primarily in the area of neuronal development. In 1974, he began research into the molecular biology, molecular and developmental ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinculin
In mammalian cells, vinculin is a membrane-cytoskeletal protein in focal adhesion plaques that is involved in linkage of integrin adhesion molecules to the actin cytoskeleton. Vinculin is a cytoskeletal protein associated with cell-cell and cell-matrix junctions, where it is thought to function as one of several interacting proteins involved in anchoring F-actin to the membrane. Discovered independently by Benny Geiger and Keith Burridge, its sequence is 20%–30% similar to α-catenin, which serves a similar function. Binding alternately to talin or α-actinin, vinculin's shape and, as a consequence, its binding properties are changed. The vinculin gene occurs as a single copy and what appears to be no close relative to take over functions in its absence. Its splice variant metavinculin (see below) also needs vinculin to heterodimerize and work in a dependent fashion. Structure Vinculin is a 117-kDa cytoskeletal protein with 1066 amino acids. The protein contains an acidi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinin
Actinin is a microfilament protein. The functional protein is an anti-parallel dimer, which cross-links the thin filaments in adjacent sarcomeres, and therefore coordinates contractions between sarcomeres in the horizontal axis. Alpha-actinin is a part of the spectrin superfamily. This superfamily is made of spectrin, dystrophin, and their homologous and isoforms. In non-muscle cells, it is found by the actin filaments and at the adhesion sites. The lattice like arrangement provides stability to the muscle contractile apparatus. Specifically, it helps bind actin filaments to the cell membrane. There is a binding site at each end of the rod and with bundles of actin filaments. The non-sarcomeric alpha-actinins, encoded by '' ACTN1'' and '' ACTN4'', are widely expressed. '' ACTN2'' expression is found in both cardiac and skeletal muscle, whereas ''ACTN3'' is limited to the latter. Both ends of the rod-shaped alpha-actinin dimer contain actin-binding domains. Six different protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Talin Protein
Talin may refer to: Places * Talin, Armenia, a city * Tálín, a municipality and village in the Czech Republic *Tallinn, capital of Estonia * Talin, Iran, a village in West Azerbaijan Province * Talin, Syria, a village in Tartus Governorate Other * Talin (protein), the protein that connects integrin to the cytoskeleton *Thaumatin Thaumatin (also known as talin) is a low-calorie sweetener and taste modifier. The protein is often used primarily for its flavor-modifying properties and not exclusively as a sweetener. The thaumatins were first found as a mixture of protein ..., a flavoring * Facundo Talín (born 1985), Argentine footballer * David Joiner (born 1958), Game programmer, hacker, going by "Talin" See also * Talen (other) * Talon (other) {{dab, geo, surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |