|
Vilsmeier–Haack Reaction
The Vilsmeier–Haack reaction (also called the Vilsmeier reaction) is the chemical reaction of a substituted amide (1) with phosphorus oxychloride and an electron-rich arene (3) to produce an aryl aldehyde or ketone (5). The reaction is named after Anton Vilsmeier and Albrecht Haack. For example, benzanilide and dimethylaniline react with phosphorus oxychloride to produce an unsymmetrical diaryl ketone. Similarly, anthracene is formylated at the 9-position. The reaction of anthracene with ''N''-methylformanilide, also using phosphorus oxychloride, gives 9-anthracenecarboxaldehyde: : Reaction mechanism The reaction of a substituted amide with phosphorus oxychloride gives a substituted chloroiminium ion (2), also called the Vilsmeier reagent. The initial product is an iminium ion (4b), which is hydrolyzed to the corresponding ketone or aldehyde In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anton Vilsmeier
Dr. Anton Vilsmeier (12 June 1894 – 12 February 1962) was a German chemist who together with Albrecht Haack discovered the Vilsmeier-Haack reaction. Early life Anton Vilsmeier was born to the mill owner, Wolfgang Vilsmeier, and his wife, Philomena, in Burgweinting, Oberpfalz. He attended the ''Volksschule'' and the '' Altes Gymnasium'' in Regensburg. During World War I, he served in the 11th Bavarian Infantry Regiment, and became a British prisoner following the Battle of the Somme, returning to Germany in November 1919. From 1920, he studied chemistry at the University of Munich, and from 1922 at the University of Erlangen, where he continued as an assistant after his studies. Career Vilsmeier discovered the aldehyde synthesis reaction which bears his name in 1926, and it was published in 1927, the year that he began to work for BASF in Ludwigshafen Ludwigshafen, officially Ludwigshafen am Rhein (; meaning "Ludwig's Port upon Rhine"), is a city in the German state o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation. Occurrence and production Coal tar, which contains around 1.5% anthracene, remains a major source of this material. Common impurities are phenanthrene and carbazole. The mineral form of anthracene is called freitalite and is related to a coal deposit. A classic laboratory method for the preparation of anthracene is by cyclodehydration of o-methyl- or o-methylene-substituted diarylketones in the so-called Elbs reaction, for example from ''o''-tolyl phenyl ketone. Reactions Reduction Reduction of anthracene with alkali metals yields the deeply colored radical anion salts M+ nthracenesup>− (M = Li, Na, K). Hydrogenation gives 9,10- dihydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Reactions
Addition (usually signified by the Plus and minus signs#Plus sign, plus symbol ) is one of the four basic Operation (mathematics), operations of arithmetic, the other three being subtraction, multiplication and Division (mathematics), division. The addition of two Natural number, whole numbers results in the total amount or ''summation, sum'' of those values combined. The example in the adjacent image shows a combination of three apples and two apples, making a total of five apples. This observation is equivalent to the Expression (mathematics), mathematical expression (that is, "3 ''plus'' 2 is Equality (mathematics), equal to 5"). Besides counting items, addition can also be defined and executed without referring to concrete objects, using abstractions called numbers instead, such as integers, real numbers and complex numbers. Addition belongs to arithmetic, a branch of mathematics. In algebra, another area of mathematics, addition can also be performed on abstract objects su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formylation Reaction
A formylation reaction in organic chemistry refers to organic reactions in which an organic compound is functionalized with a formyl group (-CH=O). The reaction is a route to aldehydes (''C''-CH=O), formamides (''N''-CH=O), and formate esters (''O''-CH=O). A reagent that delivers the formyl group is called a formylating agent. A particularly important formylation process is hydroformylation which converts alkenes to the homologated aldehyde. The conversion of benzene to benzaldehyde is the basis of the Gattermann–Koch reaction: Aromatic formylation Formylation reactions are a form of electrophilic aromatic substitution and therefore work best when the aromatic starting materials are electron-rich. Phenols are very commonly encountered as they can be readily deprotonated to form phenoxides which are excellent nucleophiles, other electron rich substrates such as mesitylene, pyrrole, or fused aromatic rings can also be expected to react. Benzene will react under aggressive con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
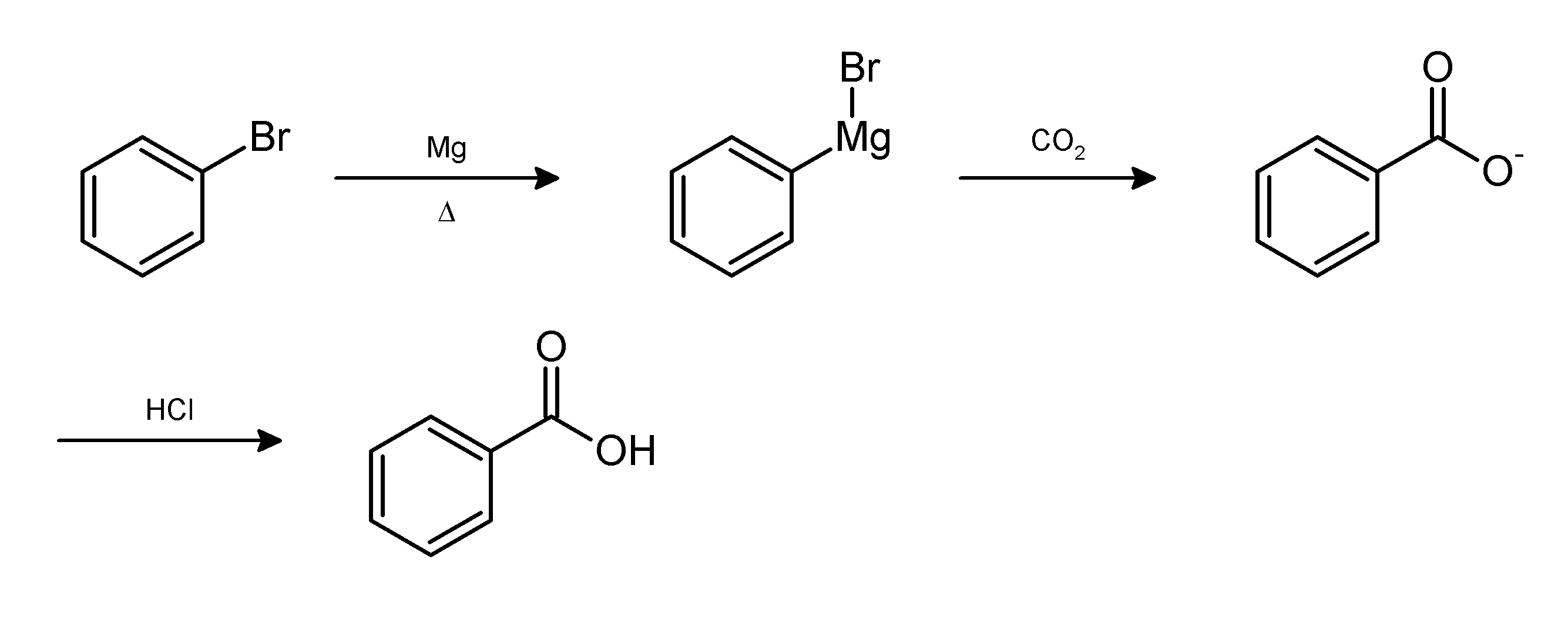
Workup (chemistry)
In chemistry, work-up refers to the series of manipulations required to isolate and purify the product(s) of a chemical reaction. Typically, these manipulations may include: * quenching a reaction to deactivate any unreacted reagents. * cooling the reaction mixture or adding an ''antisolvent'' to induce precipitation, and collecting or removing the solids by filtration, decantation, or centrifugation * removal of solvents by evaporation * separating the reaction mixture into organic and aqueous layers by liquid-liquid extraction * purification by chromatography, distillation or recrystallization For example, the Grignard reaction between phenylmagnesium bromide and carbon dioxide in the form of dry ice gives the conjugate base of benzoic acid. The desired product, benzoic acid, is obtained by the following work-up:{{cite book , title = Introduction to Organic Laboratory Techniques: A Small Scale Approach , author = Donald L. Pavia , year = 2004 , publisher = Thomson Bro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolyze
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iminium Ion
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology. Structure Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with all four substituents. The C=N distances, which are near 129 picometers in length, are shorter than C-N single bonds. Cis/trans isomers are observed. Formation Iminium cations are obtained by protonation and alkylation of imines: :RN=CR'_2 + H+ -> NH=CR'_2 :RN=CR'_2 + R''+ -> R''N=CR'_2 They also are generated by the condensation of secondary amines with ketones or aldehydes: :O=CR'_2 + R2NH + H+ 2N=CR'_2 + H2O This rapid, reversible reaction is one step in "iminium catalysis". More exotic routes to iminium cations are known, e.g. from ring-opening reactions of pyridine. Occurrence Iminium derivatives are common in biology. Pyridoxal phosphate reacts with amino acids to give iminium derivatives. Many iminium salts are en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vilsmeier Reagent
The Vilsmeier reagent is an organic compound with the formula CH3)2NCHCll. It is a salt consisting of the N,N-dimethyliminium cation ( CH3)2N=CHClsup>+) and chloride anion. Depending on the particular reaction, the anion can vary. In typical POCl3-based reactions, the anion is PO2Cl2−. The iminium cation CH3)2N=CHClsup>+ is the reactive component of interest. This iminium species is a derivative of the imidoyl chloride CH3N=CHCl. Analogues of this particular reagent are generated when tertiary amides other than DMF are treated with POCl3. The salt is a white solid that is soluble in polar organic solvents. Vilsmeier reagent is the active intermediate in the formylation reactions, the Vilsmeier reaction or Vilsmeier-Haack reaction that use mixtures of dimethylformamide Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
9-Anthracenecarboxaldehyde
Anthracene-9-carbaldehyde is the most common monoaldehyde derivative of anthracene. It is a yellow solid that is soluble in common organic solvents. It is prepared by Vilsmeier formylation of anthracene. The compound is also used as a building block for supramolecular assemblies. Hydrogenation Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate org ... of 9-anthracenecarboxaldehyde gives 9-anthracenemethanol. References Aromatic aldehydes Anthracenes {{Hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylaniline
''N'',''N''-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It consists of a tertiary amine, featuring dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow. It is an important precursor to dyes such as crystal violet. Preparation and reactions DMA was first reported in 1850 by the German chemist A. W. Hofmann, who prepared it by heating aniline and iodomethane: :C6H5NH2 + 2 CH3I → C6H5N(CH3)2 + 2 HI DMA is produced industrially by alkylation of aniline with methanol in the presence of an acid catalyst:Kahl, Thomas ''et al.'' (2007) "Aniline" in ''Ullmann's Encyclopedia of Industrial Chemistry''. John Wiley & Sons: New York. :C6H5NH2 + 2 CH3OH → C6H5N(CH3)2 + 2 H2O Similarly, it is also prepared using dimethyl ether as the methylating agent. Dimethylaniline undergoes many of the reactions expected for an aniline, being weakly basic and r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |