|
Vanadate
In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest oxidation state of +5. The complexes and are referred to as hexacyanovanadate(III) and nonachlorodivanadate(III), respectively. A simple vanadate ion is the tetrahedral orthovanadate anion, (which is also called vanadate(V)), which is present in e.g. sodium orthovanadate and in solutions of vanadium pentoxide, in strong base (pH > 13). Conventionally this ion is represented with a single double bond, however this is a resonance (chemistry), resonance form as the ion is a regular tetrahedron with four equivalent oxygen atoms. Additionally a range of polyoxovanadate ions exist which include discrete ions and "infinite" polymeric ions. There are also vanadates, such as rhodium vanadate, , which has a statistical titanium dioxide, rutile structure where the and ions randomly occupy the positions in the rutile latti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decavanadate Polyhedra
Sodium decavanadate describes any member of the family of inorganic compounds with the formula Na6[V10O28](H2O)n. These are sodium salts of the orange-colored decavanadate anion [V10O28]6−. Numerous other decavanadate salts have been isolated and studied since 1956 when it was first characterized. Preparation The preparation of decavanadate is achieved by acidifying an aqueous solution of ortho-vanadate: :10 Na3[VO4] + 24 HOAc → Na6[V10O28] + 12 H2O + 24 NaOAc The formation of decavanadate is optimized by maintaining a pH range of 4–7. Typical side products include metavanadate, [VO3]−, and hexavanadate, [V6O16]2−, ions. Structure The decavanadate ion consists of 10 fused VO6 octahedra and has D2h symmetry. The structure of Na6[V10O28]·18H2O has been confirmed with X-ray crystallography. The decavanadate anions contains three sets of equivalent V atoms (see fig. 1). These include two central VO6 octahedra (Vc) and four each peripheral tetragonal-pyramidal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthovanadate Anion
In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest oxidation state of +5. The complexes and are referred to as hexacyanovanadate(III) and nonachlorodivanadate(III), respectively. A simple vanadate ion is the tetrahedral orthovanadate anion, (which is also called vanadate(V)), which is present in e.g. sodium orthovanadate and in solutions of in strong base ( pH > 13). Conventionally this ion is represented with a single double bond, however this is a resonance form as the ion is a regular tetrahedron with four equivalent oxygen atoms. Additionally a range of polyoxovanadate ions exist which include discrete ions and "infinite" polymeric ions. There are also vanadates, such as rhodium vanadate, , which has a statistical rutile structure where the and ions randomly occupy the positions in the rutile lattice, that do not contain a lattice of cations and balancin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium
Vanadium is a chemical element; it has Symbol (chemistry), symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer (passivation (chemistry), passivation) somewhat stabilizes the free metal against further oxidation. Spain, Spanish-Mexico, Mexican scientist Andrés Manuel del Río discovered compounds of vanadium in 1801 by analyzing a new lead-bearing mineral he called "brown lead". Though he initially presumed its qualities were due to the presence of a new element, he was later erroneously convinced by French chemist Hippolyte Victor Collet-Descotils that the element was just chromium. Then in 1830, Nils Gabriel Sefström generated chlorides of vanadium, thus proving there was a new element, and named it "vanadium" after the Scandinavian goddess of beauty and fertility, Vanadís (Freyja). The name was based on the wide range of colors fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Orthovanadate
Sodium orthovanadate is the inorganic compound with the chemical formula . It forms a dihydrate . Sodium orthovanadate is a salt of the oxyanion. It is a colorless, water-soluble solid. Synthesis and structure Sodium orthovanadate is produced by dissolving vanadium(V) oxide in a solution of sodium hydroxide: : The salt features tetrahedral anion centers linked to octahedral cation sites. Condensation equilibria Like many oxometalates, orthovanadate is subject to a number of reactions, which have been analyzed by 51V NMR studies. At high pH, ions exist in equilibrium with . At lower pH's, condensation ensues to give various polyoxovanadates. Ultimately, decavanadate is formed. Biochemistry Vanadates exhibit a variety of biological activities, in part because they serve as structural mimics of phosphates. It acts as a competitive inhibitor of ATPases, alkaline and acid phosphatases, and protein-phosphotyrosine phosphatases, and its inhibitory effects can be reversed by diluti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium Pentoxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a dark yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst. The mineral form of this compound, shcherbinaite, is extremely rare, almost always found among fumaroles. A mineral trihydrate, V2O5·3H2O, is also known under the name of navajoite. Chemical properties Reduction to lower oxides Upon heating a mixture of vanadium(V) oxide and vanadium(III) oxide, comproportionation occurs to give vanadium(IV) oxide, as a deep-blue solid: :V2O5 + V2O3 → 4 VO2 The reduction can also be effected by oxalic acid, carbon monoxide, and sulfur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdate
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of +6: . Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed ''polyoxometalates'', and because they contain only one type of metal atom are often called ''isopolymetalates''. The discrete molybdenum oxyanions range in size from the simplest , found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, , , and ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten. Examples of molybdate anions Examples of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
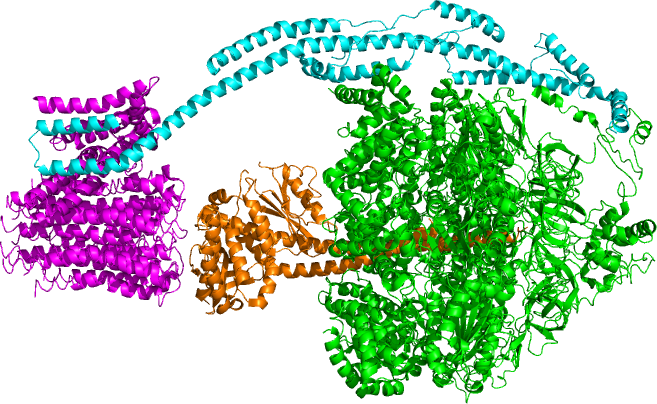
P-type ATPase
The P-type ATPases, also known as E1-E2 ATPases, are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. P-type ATPases are α-helical bundle primary transporters named based upon their ability to catalyze auto- (or self-) phosphorylation (hence P) of a key conserved aspartate residue within the pump and their energy source, adenosine triphosphate (ATP). In addition, they all appear to interconvert between at least two different conformations, denoted by E1 and E2. P-type ATPases fall under the P-type ATPase (P-ATPase) SuperfamilyTC# 3.A.3 which, as of early 2016, includes 20 different protein families. Most members of this transporter superfamily catalyze cation uptake and/or efflux, however one subfamily, the flippases,TC# 3.A.3.8 is involved in flipping phospholipids to maintain the asymmetric nature of the biomembrane. In humans, P-type ATPases serve as a basis for nerve impulses, relaxation of muscles, secre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Vascular System
''The'' is a grammatical article in English, denoting nouns that are already or about to be mentioned, under discussion, implied or otherwise presumed familiar to listeners, readers, or speakers. It is the definite article in English. ''The'' is the most frequently used word in the English language; studies and analyses of texts have found it to account for seven percent of all printed English-language words. It is derived from gendered articles in Old English which combined in Middle English and now has a single form used with nouns of any gender. The word can be used with both singular and plural nouns, and with a noun that starts with any letter. This is different from many other languages, which have different forms of the definite article for different genders or numbers. Pronunciation In most dialects, "the" is pronounced as (with the voiced dental fricative followed by a schwa) when followed by a consonant sound, and as (homophone of the archaic pronoun ''thee'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondrial ATPase
ATP synthase is an enzyme that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is: * ADP + Pi + 2H+out ATP + H2O + 2H+in ATP synthase lies across a cellular membrane and forms an aperture that protons can cross from areas of high concentration to areas of low concentration, imparting energy for the synthesis of ATP. This electrochemical gradient is generated by the electron transport chain and allows cells to store energy in ATP for later use. In prokaryotic cells ATP synthase lies across the plasma membrane, while in eukaryotic cells it lies across the inner mitochondrial membrane. Organisms capable of photosynthesis also have ATP synthase across the thylakoid membrane, which in plants is located in the chloroplast and in cyanobacteria is located in the cytoplasm. Eukaryotic ATP synthases are F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little net". It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. There are two types of ER that share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of Cell (biology), cells contain different ratios of the two types of ER dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |