|
Van Der Waals Equation
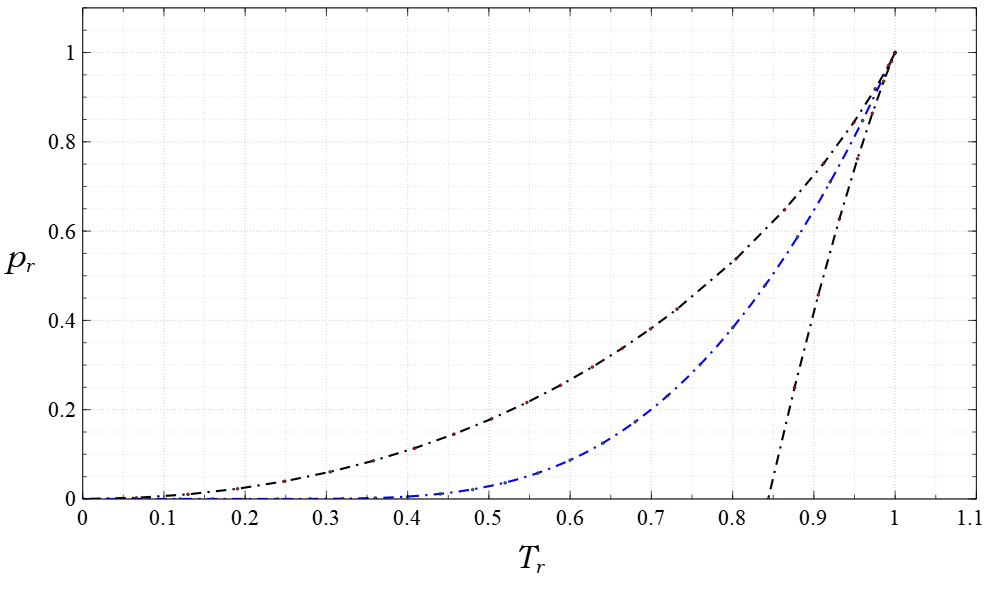
The van der Waals equation is a mathematical formula that describes the behavior of real gases. It is an equation of state that relates the pressure, volume, Avogadro's law, number of molecules, and temperature in a fluid. The equation modifies the ideal gas law in two ways: first, it considers particles to have a finite diameter (whereas an ideal gas consists of point particles); second, its particles interact with each other (unlike an ideal gas, whose particles move as though alone in the volume). The equation is named after Dutch physicist Johannes Diderik van der Waals, who first derived it in 1873 as part of his doctoral thesis. Van der Waals based the equation on the idea that fluids are composed of discrete particles, which few scientists believed existed. However, the equation accurately predicted the behavior of a fluid around its Critical point (thermodynamics), critical point, which had been discovered a few years earlier. Its qualitative and quantitative agreement w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Real Gas
Real gases are non-ideal gases whose molecules occupy space and have interactions; consequently, they do not adhere to the ideal gas law. To understand the behaviour of real gases, the following must be taken into account: * compressibility effects; *variable specific heat capacity; *van der Waals forces; *non-equilibrium thermodynamic effects; *issues with molecular dissociation and elementary reactions with variable composition For most applications, such a detailed analysis is unnecessary, and the ideal gas approximation can be used with reasonable accuracy. On the other hand, real-gas models have to be used near the condensation point of gases, near critical points, at very high pressures, to explain the Joule–Thomson effect, and in other less usual cases. The deviation from ideality can be described by the compressibility factor Z. Models Van der Waals model Real gases are often modeled by taking into account their molar weight and molar volume RT = \left(p + \frac\r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Theory Of Gases
The kinetic theory of gases is a simple classical model of the thermodynamic behavior of gases. Its introduction allowed many principal concepts of thermodynamics to be established. It treats a gas as composed of numerous particles, too small to be seen with a microscope, in constant, random motion. These particles are now known to be the atoms or molecules of the gas. The kinetic theory of gases uses their collisions with each other and with the walls of their container to explain the relationship between the macroscopic properties of gases, such as volume, pressure, and temperature, as well as transport properties such as viscosity, thermal conductivity and mass diffusivity. The basic version of the model describes an ideal gas. It treats the collisions as perfectly elastic and as the only interaction between the particles, which are additionally assumed to be much smaller than their average distance apart. Due to the time reversibility of microscopic dynamics ( microsco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion. In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Robert and Halliday, David (1960) ''Physics'', Section 7-5, Wiley International Edition The kinetic energy of an object is equal to the work, or force ( F) in the direction of motion times its displacement ( s), needed to accelerate the object from rest to its given speed. The same amount of work is done by the object when decelerating from its current speed to a state of rest. The SI unit of energy is the joule, while the English unit of energy is the foot-pound. In relativistic mechanics, \fracmv^2 is a good approximation of kinetic energy only when ''v'' is much less than the speed of light. History and etymology The adjective ''kinetic'' has its roots in the Greek word κίνησις ''kinesis'', meaning "motion". The dichoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rudolf Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he gave the theory of heat a truer and sounder basis. His most important paper, "On the Moving Force of Heat", published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat. Life Clausius was born in Köslin (now Koszalin, Poland) in the Province of Pomerania in Prussia. His father was a Protestant pastor and school inspector, and Rudolf studied in the school of his father. In 1838, he went to the Gymnasium in Stettin. Clausius graduated from the University of Berlin in 1844 where he had studied mathematics and physics since 1840 with, among others, Gustav Magnus, Peter Gusta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrodynamica
''Hydrodynamica, sive de Viribus et Motibus Fluidorum Commentarii'' (Latin for ''Hydrodynamics, or commentaries on the forces and motions of fluids'') is a book published by Daniel Bernoulli in 1738. The title of this book eventually christened the field of fluid mechanics as hydrodynamics. This book introduced the Bernoulli's principle, stating the first form of conservation of energy in fluid dynamics. Description The book deals with fluid mechanics and is organized around preliminary versions of the conservation of energy, as received from Christiaan Huygens's formulation of Vis viva, ''vis viva'' (Latin for living forces). The book describes the theory of water flowing through a tube and of water flowing from a hole in a container. In doing so, Bernoulli explained the nature of hydrodynamic pressure and discovered the role of loss of ''vis viva'' in fluid flow, which would later be known as the Bernoulli principle. The book also discusses hydraulic machines and introduces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daniel Bernoulli
Daniel Bernoulli ( ; ; – 27 March 1782) was a Swiss people, Swiss-France, French mathematician and physicist and was one of the many prominent mathematicians in the Bernoulli family from Basel. He is particularly remembered for his applications of mathematics to mechanics, especially fluid mechanics, and for his pioneering work in probability and statistics. His name is commemorated in the Bernoulli's principle, a particular example of the conservation of energy, which describes the mathematics of the mechanism underlying the operation of two important technologies of the 20th century: the carburetor and the aeroplane wing. Early life Daniel Bernoulli was born in Groningen (city), Groningen, in the Netherlands, into a Bernoulli family, family of distinguished mathematicians.Murray Rothbard, Rothbard, MurrayDaniel Bernoulli and the Founding of Mathematical Economics, ''Mises Institute'' (excerpted from ''An Austrian Perspective on the History of Economic Thought'') The Bernou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boyle's Law
Boyle's law, also referred to as the Boyle–Mariotte law or Mariotte's law (especially in France), is an empirical gas laws, gas law that describes the relationship between pressure and volume of a confined gas. Boyle's law has been stated as: The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of substance, amount of gas remain unchanged within a closed system.Levine (1978) p. 12 gives the original definition. Mathematically, Boyle's law can be stated as: or where is the pressure of the gas, is the volume of the gas, and is a Constant (mathematics), constant for a particular temperature and amount of gas. Boyle's law states that when the temperature of a given mass of confined gas is constant, the product of its pressure and volume is also constant. When comparing the same substance under two different sets of conditions, the law can be expressed as: P_1 V_1 = P_2 V_2. showi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isaac Newton
Sir Isaac Newton () was an English polymath active as a mathematician, physicist, astronomer, alchemist, theologian, and author. Newton was a key figure in the Scientific Revolution and the Age of Enlightenment, Enlightenment that followed. His book (''Mathematical Principles of Natural Philosophy''), first published in 1687, achieved the Unification of theories in physics#Unification of gravity and astronomy, first great unification in physics and established classical mechanics. Newton also made seminal contributions to optics, and Leibniz–Newton calculus controversy, shares credit with German mathematician Gottfried Wilhelm Leibniz for formulating calculus, infinitesimal calculus, though he developed calculus years before Leibniz. Newton contributed to and refined the scientific method, and his work is considered the most influential in bringing forth modern science. In the , Newton formulated the Newton's laws of motion, laws of motion and Newton's law of universal g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld (; 5 December 1868 – 26 April 1951) was a German Theoretical physics, theoretical physicist who pioneered developments in Atomic physics, atomic and Quantum mechanics, quantum physics, and also educated and mentored many students for the new era of theoretical physics. He served as doctoral advisor and Postdoctoral researcher, postdoc advisor to seven Nobel Prize winners and supervised at least 30 other famous physicists and chemists. Only J. J. Thomson's record of mentorship offers a comparable list of high-achieving students. He introduced the second quantum number, azimuthal quantum number, and the third quantum number, magnetic quantum number. He also introduced the fine-structure constant and pioneered X-ray wave theory. Early life and education Sommerfeld was born in 1868 to a family with deep ancestral roots in Prussia. His mother Cäcilie Matthias (1839–1902) was the daughter of a Potsdam builder. His father Franz Sommerfeld (1820� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Sophus Epstein
Paul Sophus Epstein (; March 20, 1883 – February 8, 1966) was a Russian-American mathematical physicist. He was known for his contributions to fluid dynamics and to the development of quantum mechanics. Early life and studies Paul Epstein's parents, Siegmund Simon Epstein and Sarah Sophia (Lurie) Epstein were of a middle class Jewish family. He said that his mother recognized his potential at the age of four years and predicted that he would be a mathematician. He went to the Hochschule in Minsk, and from 1901 to 1905 studied mathematics and physics at the Imperial University of Moscow under Pyotr Nikolaevich Lebedev. In 1909 he graduated, and became a Privatdozent at the University of Moscow. In 1910 he went to Munich, Germany, to do research under Arnold Sommerfeld, who was his advisor, and Epstein was granted a Ph.D. on a problem in the theory of diffraction of electromagnetic waves. from the Technische Universität München, in 1914. Career At the outbreak of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: ) under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boiling, boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one Atmosphere (unit), atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |