|
Uranium Hexachloride
Uranium hexachloride is the inorganic compound with the formula . It features uranium in the +6 oxidation state. hydrolyzes readily but is stable under inert atmosphere. It is soluble in carbon tetrachloride (). It is a multi-luminescent dark green or black solid with a vapor pressure between 1-3 mmHg at 373.15 K. Structure and bonding Uranium hexachloride has an octahedral geometry, with point group Oh. Its lattice (dimensions: 10.95 ± 0.02 Å x 6.03 ± 0.01 Å) is hexagonal in shape with three molecules per cell; the average theoretical U-Cl bond is 2.472 Å long (the experimental U-Cl length found by X-ray diffraction is 2.42 Å), and the distance between two adjacent chlorine atoms is 3.65 Å. Chemical properties is stable up to temperatures between 120 °C and 150 °C. The decomposition of results in a solid phase transition from one crystal form of to another more stable form. It decomposes as follows: : The activation energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Hexafluoride
Uranium hexafluoride, sometimes called hex, is the inorganic compound with the formula . Uranium hexafluoride is a volatile, white solid that is used in enriching uranium for nuclear reactors and nuclear weapons. Preparation Uranium dioxide is converted with hydrofluoric acid (HF) to uranium tetrafluoride: : The resulting is subsequently oxidized with fluorine to give the hexafluoride: : In samples contaminated with uranium trioxide, uranyl fluoride, an oxyfluoride compound is produced in the HF step: : which can be fluorinated to produce the same product, uranium hexafluoride. : The fluorination step in both reactions above are highly exothermic. Properties Physical properties At atmospheric pressure, sublimes at 56.5 °C. The solid-state structure was determined by neutron diffraction at 77 K and 293 K.J. C. Taylor, P. W. Wilson, J. W. Kelly: „The structures of fluorides. I. Deviations from ideal symmetry in the structure of crystalline UF6: a neutron diffract ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, meaning they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are also called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Trioxide
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph is amorphous UO3. Production and use There are three methods to generate uranium trioxide. As noted below, two are used industrially in the reprocessing of nuclear fuel and uranium enrichment. # U3O8 can be oxidized at 500 °C with oxygen. Note that above 750 °C even in 5 atm O2 UO3 decomposes into U3O8. # Uranyl nitrate, UO2(NO3)2·6H2O can be heated to yield UO3. This occurs during the reprocessing of nuclear fuel. Fuel rods are dissolved in HNO3 to separate uranyl nitrate from plutonium and the fission products (the PUREX method). The pure uranyl nitrate is converted to solid UO3 by heating at 400 °C. After reduction with hydrogen (with other inert gas present) to uranium dioxide, the uranium can be used in new MOX fuel rod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Trichloride
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive towards water. Production and structure Boron reacts with halogens to give the corresponding trihalides. Boron trichloride is, however, produced industrially by chlorination reaction, chlorination of boron trioxide, boron oxide and carbon at 501 °C. :B2O3 + 3 C + 3 Cl2 → 2 BCl3 + 3 CO The carbothermic reaction is analogous to the Kroll process for the conversion of titanium dioxide to titanium tetrachloride. One consequence of this synthesis route is that samples of boron trichloride are often contaminated with phosgene. In the laboratory BCl3 can be prepared by treating with AlCl3 with boron trifluoride, BF3, a halide exchange reaction. BCl3 is a trigonal planar molecule like the other boron trihalides. The B–Cl bond length is 175 pm. A degree of π-bonding has been proposed to explain the short B− Cl distance, although t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Pentafluoride
Uranium pentafluoride is the inorganic compound with the chemical formula UF5. It is a pale yellow paramagnetic solid. The compound has attracted interest because it is related to uranium hexafluoride, which is widely used to produce uranium fuel. It crystallizes in two polymorphs, called α- and β-UF5. Synthesis and structure Uranium pentafluoride is an intermediate in the conversion of uranium tetrafluoride to volatile UF6: :2UF4 + F2 → 2UF5 :2UF5 + F2 → 2UF6 It can be produced by reduction of the hexafluoride with carbon monoxide at elevated temperatures. : 2UF6 + CO → 2UF5 + COF2 Other reducing agents have been examined. The α form is a linear coordination polymer consisting of chains of octahedral uranium centers in which one of the five fluoride anion forms a bridge to the next uranium atom. The structure is reminiscent of that for vanadium pentafluoride. In the β form, the uranium centers adopt a square antiprismatic structure. The β polymorp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
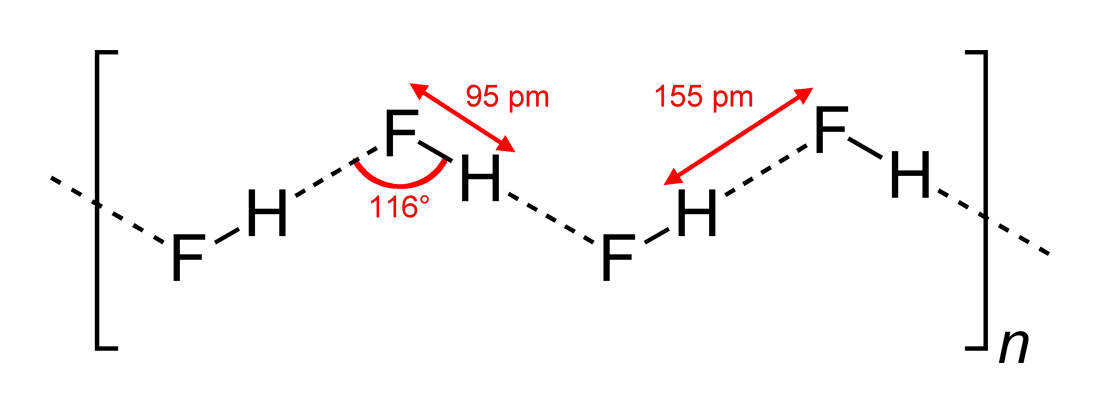
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freon 113
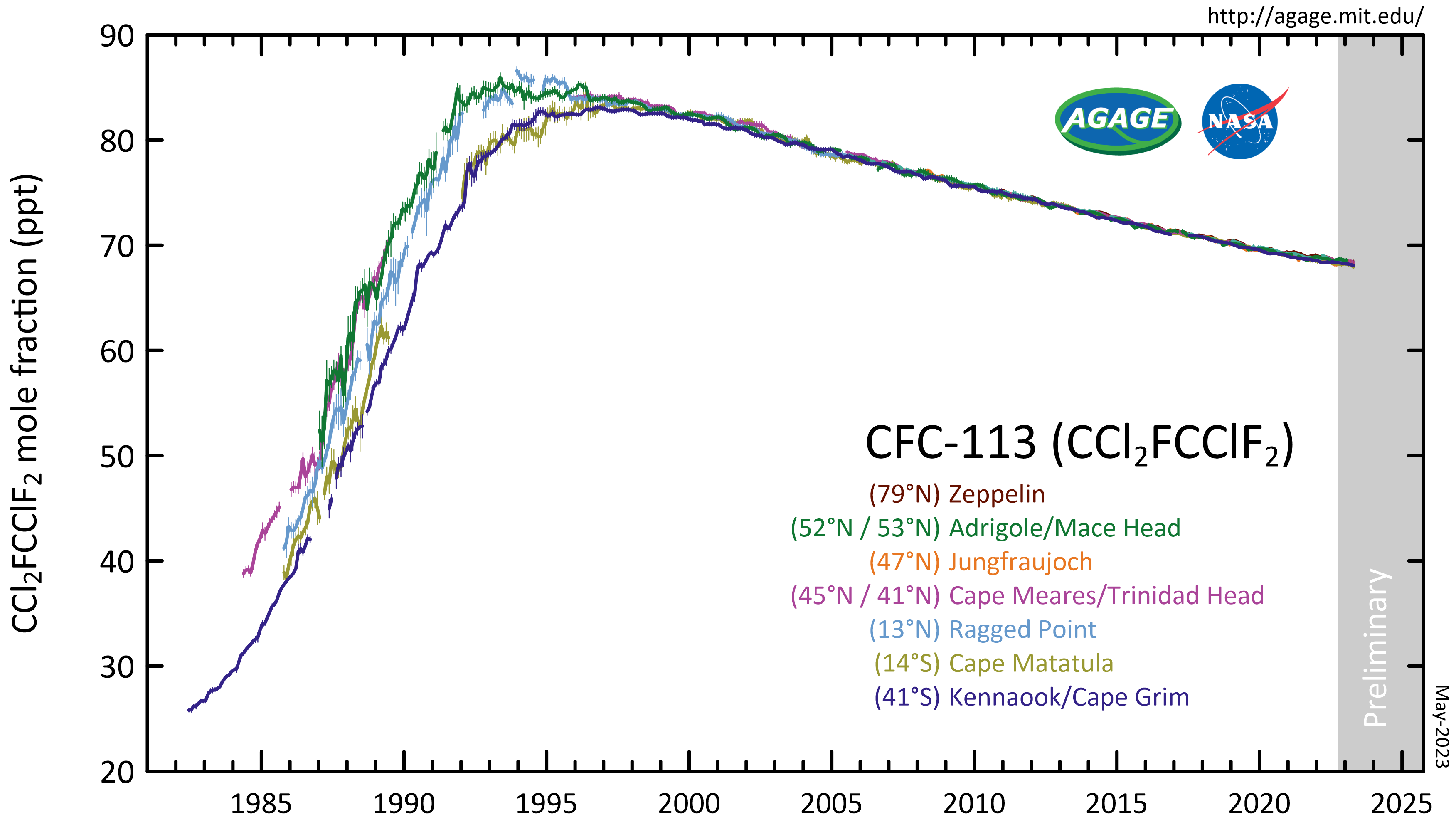
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane (often abbreviated as TCTFE) or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Production CFC-113 can be prepared from hexachloroethane and hydrofluoric acid: : This reaction may require catalysts such as antimony, chromium, iron and alumina at high temperatures. Another synthesis method uses HF on tetrachloroethylene instead. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It may remain in the Atmosphere of Earth, atmosphere up to 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (UV, sunlight in the 190-225 nm range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insoluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |