|
Trivalent Group 14 Radicals
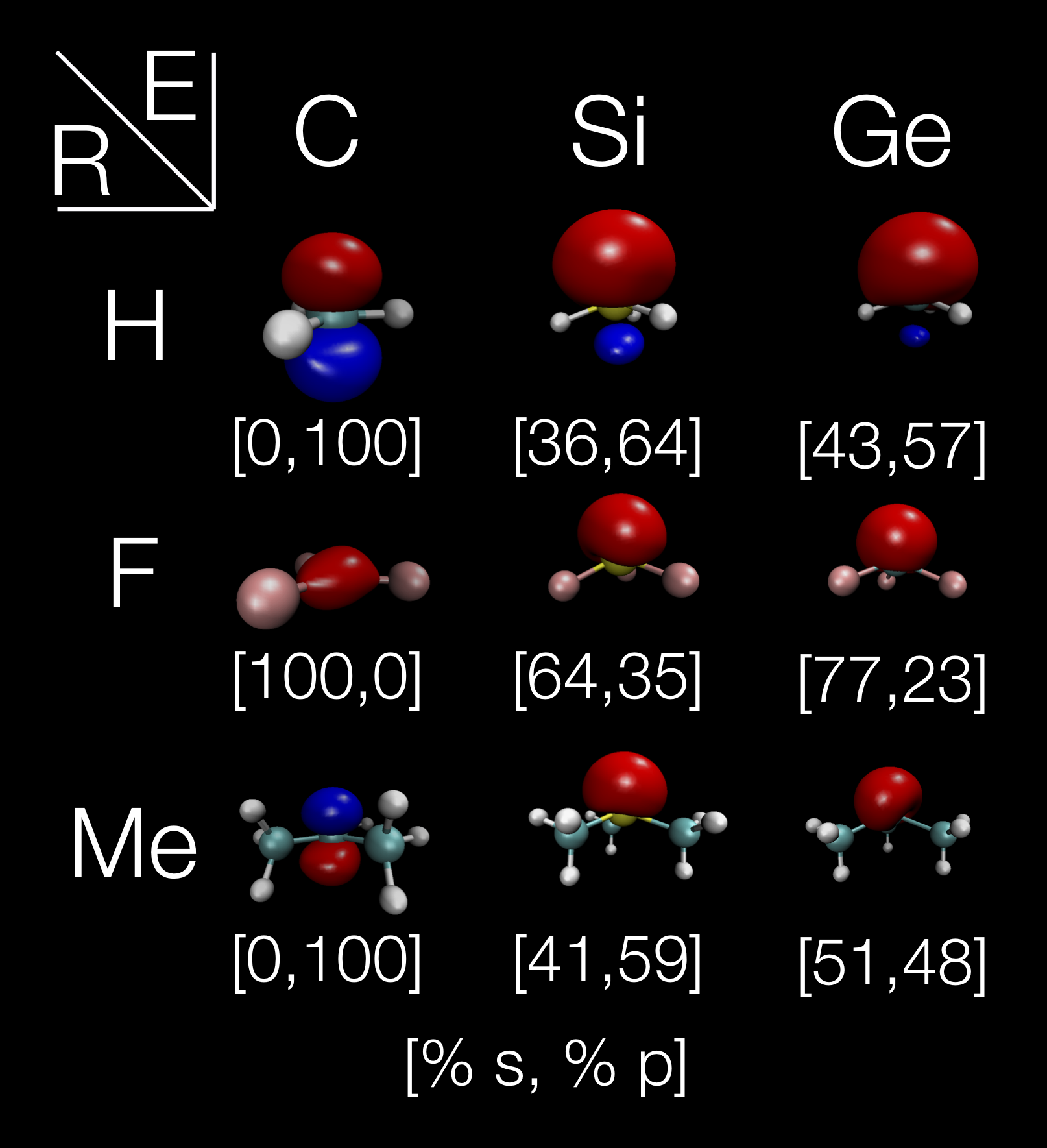
A trivalent group 14 radical (also known as a ''trivalent tetrel radical'') is a molecule that contains a group 14 element (E = C, Si, Ge, Sn, Pb) with three bonds and a free radical, having the general formula of R3E•. Such compounds can be categorized into three different types, depending on the structure (or equivalently the orbital in which the unpaired electron resides) and the energetic barrier to inversion. A molecule that remains rigidly in a pyramidal structure has an electron in a sp3 orbital is denoted as ''Type A''. A structure that is pyramidal, but flexible, is denoted as ''Type B''. And a planar structure with an electron that typically would reside in a pure p orbital is denoted as ''Type C''. The structure of such molecules has been determined by probing the nature of the orbital that the unpaired electron resides in using spectroscopy, as well as directly with X-ray methods. Trivalent tetrel radicals tend to be synthesized from their tetravalent counterparts (i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrel Radical NBO
The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block. In modern IUPAC notation, it is called group 14. In the field of semiconductor physics, it is still universally called group IV. The group was once also known as the tetrels (from the Greek word ''tetra'', which means four), stemming from the Roman numeral IV in the group names, or (not coincidentally) from the fact that these elements have four valence electrons (see below). They are also known as the crystallogens or adamantogens. Characteristics Chemical Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior: Each of the elements in this group has 4 electrons in its outer shell. An isolated, neutral group 14 atom has the s2 p2 configuration in the ground state. These elements, especial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Group
The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block. In modern IUPAC notation, it is called group 14. In the field of semiconductor physics, it is still universally called group IV. The group was once also known as the tetrels (from the Greek word ''tetra'', which means four), stemming from the Roman numeral IV in the group names, or (not coincidentally) from the fact that these elements have four valence electrons (see below). They are also known as the crystallogens or adamantogens. Characteristics Chemical Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior: Each of the elements in this group has 4 electrons in its outer shell. An isolated, neutral group 14 atom has the s2 p2 configuration in the ground state. These elements, esp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing. Ageing Ailments of unknown cause Biogerontology Biological processes Causes of death Cellular processes Gerontology Life extension Metabolic disorders Metabolism Old age Time in life Wikipedia categories named after diseases and disorders {{CatAutoTOC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethyl Radical
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited. Preparation and properties It can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid-type dimer 3 (Gomberg's dimer). In benzene the concentration of the radical is 2%. Solutions containing the radical are yellow; when the temperature of the solution is raised, the yellow color becomes more intense as the equilibrium is shifted in favor of the radical (in accordance with Le Chatelier's principle). When exposed to air, the radical rapidly oxidizes to the peroxide, and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with iodine to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira ( ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/ MEDLINE/ PubMed, and the Science Citation Index Expanded. According to the '' Journal Citation Reports'', the journal has a 2021 impact factor of 16.383. Editors-in-chief The following people are or have been editor-in-chief: * 1879–1880 – Hermann Endemann * 1880–1881 – Gideon E. Moore * 1881–1882 – Hermann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Examples * Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: ::CaCO3 → CaO + CO2 :The reaction is used to make quick lime, which is an industrially important product. :A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance-enhanced Multiphoton Ionization
Resonance-enhanced multiphoton ionization (REMPI) is a technique applied to the spectroscopy of atoms and small molecules. In practice, a tunable laser can be used to access an excited intermediate state. The selection rules associated with a two-photon or other multiphoton photoabsorption are different from the selection rules for a single photon transition. The REMPI technique typically involves a resonant single or multiple photon absorption to an electronically excited intermediate state followed by another photon which ionizes the atom or molecule. The light intensity to achieve a typical multiphoton transition is generally significantly larger than the light intensity to achieve a single photon photoabsorption. Because of this, a subsequent photoabsorption is often very likely. An ion and a free electron will result if the photons have imparted enough energy to exceed the ionization threshold energy of the system. In many cases, REMPI provides spectroscopic information th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EPR Tetrel
EPR may refer to: Science and technology * EPR (nuclear reactor), European Pressurised-Water Reactor * EPR paradox (Einstein–Podolsky–Rosen paradox), in physics * Earth potential rise, in electrical engineering * East Pacific Rise, a mid-oceanic ridge * Electron paramagnetic resonance * Engine pressure ratio,of a jet engine * Ethylene propylene rubber * Yevpatoria RT-70 radio telescope (Evpatoria planetary radar) * Bernays–Schönfinkel class or effectively propositional, in mathematical logic * Endpoint references in Web addressing * Ethnic Power Relations, dataset of ethnic groups * ePrivacy Regulation (ePR), proposal for the regulation of various privacy-related topics, mostly in relation to electronic communications within the European Union Medicine * Enhanced permeability and retention effect, a controversial concept in cancer research * Emergency Preservation and Resuscitation, a medical procedure * Electronic patient record Environment * UNECE Environmental Perfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Paramagnetic Resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford. Theory Origin of an EPR signal Every electron has a magnetic moment and spin quantum number s = \tfrac , with magnetic components m_\mathrm = + \tfrac or m_\mathrm = - \tfrac . In the presence of an external magnetic field with strength B_\mathrm , the electron's magnetic moment aligns itself either antiparallel ( m_\mathrm = - \tfrac ) or parallel ( m_\mathrm = + \tfrac ) to the fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudo Jahn–Teller Effect
The pseudo Jahn–Teller effect (PJTE), occasionally also known as second-order JTE, is a direct extension of the Jahn–Teller effect (JTE) where spontaneous symmetry breaking in polyatomic systems (molecules and solids) occurs even in nondegenerate electronic states under the influence of sufficiently low-lying excited states of appropriate symmetry. "The pseudo Jahn–Teller effect is the only source of instability and distortions of high-symmetry configurations of polyatomic systems in nondegenerate states, and it contributes significantly to the instability in degenerate states". History In their early 1957 paper on the (what is now called) pseudo Jahn–Teller effect (PJTE), Öpik and Pryce showed that a small splitting of the degenerate electronic term does not necessarily remove the instability and distortion of the polyatomic system induced by the Jahn–Teller effect (JTE), provided the splitting is sufficiently small (the two split states remain “pseudo degenerate” ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |