|
Triphenylmethyl Hexafluorophosphate
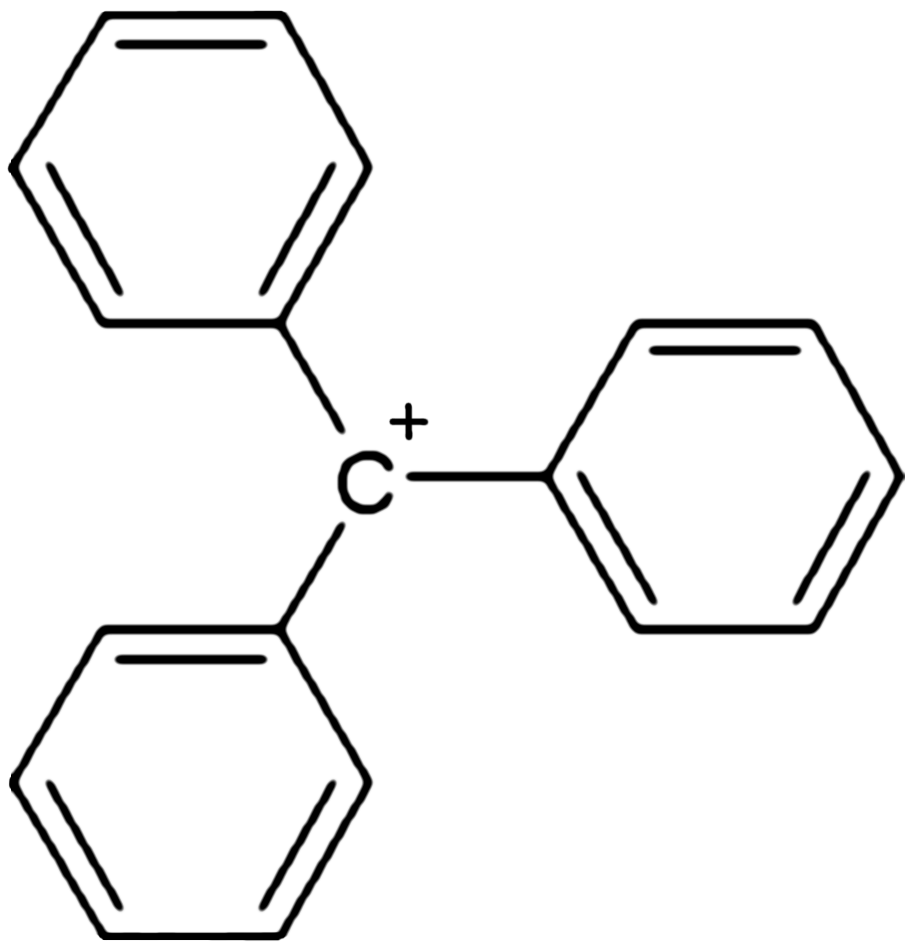
Triphenylmethyl hexafluorophosphate (also triphenylcarbenium hexafluorophosphate, trityl hexafluorophosphate, or tritylium hexafluorophosphate) is an organic salt with the formula , consisting of the triphenylcarbenium cation and the hexafluorophosphate anion . Triphenylmethyl hexafluorophosphate is a brown powder that hydrolyzes readily to triphenylmethanol. It is used as a catalyst and reagent in organic syntheses. Preparation Triphenylmethyl hexafluorophosphate can be prepared by combining silver hexafluorophosphate with triphenylmethyl chloride: : A second method involves protonolysis of triphenylmethanol: : Structure and reactions Triphenylmethyl hexafluorophosphate readily hydrolyzes, in a reaction that is the reverse of one of its syntheses: : Triphenylmethyl hexafluorophosphate has been used for abstracting hydride () from organic compounds. Treatment of metal-alkene and diene complexes one can generate allyl and pentadienyl complexes, respectively. Tripheny ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions ( anions), which results in a compound with no net electric charge (electrically neutral). The constituent ions are held together by electrostatic forces termed ionic bonds. The component ions in a salt can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic, such as ammonium () and carbonate () ions in ammonium carbonate. Salts containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases, such as sodium hydroxide and potassium oxide. Individual ions within a salt usually have multiple near neighbours, so they are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Salts usually form crystalline structures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Complex
Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures. Examples The allyl ligand is commonly in organometallic chemistry. Usually, allyl ligands bind to metals via all three carbon atoms, the hapticity, η3-binding mode. The η3-allyl group is classified as an LX-type ligand in the Green LXZ Covalent bond classification method, ligand classification scheme, serving as a 3e– donor using neutral electron counting and 4e– donor using ionic electron counting. Scope Commonly, allyl ligands occur in mixed ligand complexes. Examples include (η3-allyl)Mn(CO)4 and Cyclopentadienyl allyl palladium, CpPd(allyl). Substituents on the allyl group are also common, e.g. 2-methallyl. Homoleptic complexes * bis(allyl)nickel * bis(allyl)palladium * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocations
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. Among the simplest carbocations are the methenium (a carbenium ion), methanium (a carbonium ion), acylium ions , and vinyl cations. Until the early 1970s, carbocations were called ''carbonium ions''. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called ' non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. However, others have more narrowly defined the term 'carbonium ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Compounds
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's rule. Aromatic compounds have the following general properties: * Typically unreactive * Often non polar and hydrophobic * High carbon-hydrogen ratio * Burn with a strong sooty yellow flame, due to high C:H ratio * Undergo electrophilic substitution reactions and nucleophilic aromatic substitutions Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethyl Chloride
Triphenylmethyl chloride or trityl chloride (TrCl) is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group. Preparation Triphenylmethyl chloride is commercially available. It may be prepared by the reaction of triphenylmethanol with acetyl chloride, or by the Friedel–Crafts alkylation of benzene with carbon tetrachloride to give the trityl chloride-aluminium chloride adduct, which is then hydrolyzed. Reactions Triphenylmethylsodium can be prepared from trityl chloride dissolved in an aprotic solvent and sodium: :(C6H5)3CCl + 2 Na → (C6H5)3CNa + NaCl Reaction with silver hexafluorophosphate gives triphenylmethyl hexafluorophosphate. Trityl chloride reacts with zinc in nonpolar solvents (e.g. benzene) to form Gomberg's dimer.{{cite journal , last1=Gomberg , first1=M. , title=An Instance of Trivalent Carbon: Triphenylmethyl , journal=Journal of the American Chemical Society , date=1900 , volum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethanol
Triphenylmethanol (also known as triphenylcarbinol and TrOH) is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. In strongly acidic solutions, it produces an intensely yellow color, due to the formation of a stable "trityl" carbocation. Many derivatives of triphenylmethanol are important dyes. History After the German chemist August Kekulé and his Belgian student Antoine Paul Nicolas Franchimont (1844–1919) first synthesized triphenylmethane in 1872, the Russian doctoral student Walerius Hemilian (1851–1914) first synthesized triphenylmethanol in 1874 by reacting triphenylmethyl bromide with water as well as by oxidizing triphenylmethane. Structure and properties Triphenylmethanol features three phenyl (Ph) rings and an alcohol group bound to a central tetrahedral carbon atom. All three C–Ph bonds are typical of ''sp''3-''sp''2 carbon-carbon bonds with lengths of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethane
Triphenylmethane or triphenyl methane (sometimes also known as Tritan), is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical). Preparation Triphenylmethane was first synthesized in 1872 by the German chemist August Kekulé and his Dutch student Antoine Paul Nicolas Franchimont (1844–1919) by heating diphenylmercury (Hg(C6H5)2, ''Quecksilberdiphenyl'') with benzal chloride (C6H5CHCl2, ''Benzylenchlorid''). Triphenylmethane can be synthesized by Friedel–Crafts reaction from benzene and chloroform with aluminium chloride catalyst: :3 C6H6 + CHCl3 → Ph3CH + 3 HCl Alternatively, benzene may re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylcarbenium
In chemistry, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula or , consisting of a carbon atom with a positive charge connected to three phenyl groups. It is a charged version of the triphenylmethyl radical . The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride that do not contain the cation. Triphenylcarbenium is a relatively stable carbenium ion, because the positive charge is partially distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom). Derivatives The cation exists in important chemical reagents and catalysts such as triphenylmethyl hexafluorophosphate . Related salts are known with diverse anions including tetrafluoroborate (), hexachloroantimonate (), and perchlorate (). This and other simil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethyl Radical
The triphenylmethyl radical (often shortened to trityl radical after 1927 suggestion by Burckhardt Helferich, Helferich et al.) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical (chemistry), radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited. Preparation and properties The triphenylmethyl radical can be prepared by homolysis (chemistry), homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid-type Dimer (chemistry), dimer 3 (Gomberg's dimer). In benzene the concentration of the radical is 2%. Solutions containing the radical are yellow; when the temperature of the solution is raised, the yellow color becomes more intense as the equilibrium is shifted in favor of the radical rather than the colorless dimer, in accordance with Le Chatelier's princi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylmethyl Perchlorate
The triphenylmethyl radical (often shortened to trityl radical after 1927 suggestion by Helferich et al.) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited. Preparation and properties The triphenylmethyl radical can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid-type dimer 3 (Gomberg's dimer). In benzene the concentration of the radical is 2%. Solutions containing the radical are yellow; when the temperature of the solution is raised, the yellow color becomes more intense as the equilibrium is shifted in favor of the radical rather than the colorless dimer, in accordance with Le Chatelier's principle. The triphenylmethyl radical exhibits green photoluminescence. Further react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentadienyl Complex
In organic chemistry, pentadiene is any hydrocarbon with an open chain of five carbons, connected by two single bonds and two double bonds. All those compounds have the same molecular formula . The inventory of pentadienes include: * 1,2-pentadiene, or ethyl allene, .James R. Durig, Stephen Bell, Gamil A. Guirgis (1996): "Infrared and Raman spectra, conformational stability, ab initio calculations and vibrational assignment for 1,2-pentadiene (ethyl allene)". ''Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy'', volume 52, issue 14, pages 1843-1859. It and 2,3-pentadiene are the least common isomers of pentadiene. * 1,3-pentadiene, with two isomers:Aldo Priola, Sebastiano Cesca, Giuseppe Ferraris, and Mario Bruzzone (1975): "Relative reactivity of cis‐ and trans‐1,3‐pentadiene in the cationic copolymerization with isobutene". ''Die Makromolekulare Chemie'', volume 176, issue 7, pages 1969-1981. S. Boue and Rangaswamy Srinivasan (1970): "Differences in rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Alkene Complex
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. The inventory is large.Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products. Monoalkenes Complexes of ethylene are particularly common. Examples include Zeise's salt (see figure), Rh2Cl2(C2H4)4, Cp*2Ti(C2H4), and . Homoleptic alkene-complexes are well known but often are highly reactive. Examples include Ni(C2H4)3, o(C2H4)4sup>−, and e(C2H4)4sup>2−. Substituted monoalkenes are common ligands. Cyclooctene is found in chlorobis(cyclooctene)rhodium dimer. Alkenes with electron-withdrawing groups commonly bind strongly to low-valent metals. Examples of such ligands are TCNE, tetrafluoroethylene, maleic anhydride, and esters of fumaric acid. These acceptors form adducts with many zero-valent metals. Dienes, trienes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |