|
Trimethylhexamethylenediamine
Trimethylhexamethylenediamine is the name used to refer to a mixture of two isomers of trimethyl- 1,6-hexanediamine. The mixture is used as a monomer in nylon TMDT. It is available commercially under the trade name Vestamin TMD from the company Evonik Industries. Trimethylhexamethylenediamine is synthesized from isophorone. Isophorone is reduced by hydrogenation to the trimethylcyclohexanol, which is then oxidized with nitric acid (in the same fashion as adipic acid is synthesized from cyclohexane Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohex ...). The diacid is converted to the diamine via the dinitrile. Uses TMD is used as a component in certain curing agents for epoxy resins. References Diamines B {{amine-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isophorone
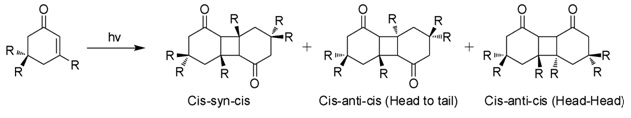
Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially. Structure and reactivity Isophorone undergoes reactions characteristic of an α,β-unsaturated ketone. Hydrogenation gives the cyclohexanone derivative. Epoxidation with basic hydrogen peroxide affords the oxide. Isophorone is degraded by attack of hydroxyl radicals. Photodimerization When exposed to sunlight in aqueous solutions, isophorone undergoes 2+2 photocycloaddition to give three isomeric photodimers (Figure). These "diketomers" are cis-syn-cis, head to tail (HT), cys-anti-cys (HT), and head-head (HH). The formation of HH photodimers is favored over HT photodimers with increasing polarity of the medium. Natural Occurrence Isophorone occurs naturally in cranberries. Synthesis Isophorone is pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,6-hexanediamine
Hexamethylenediamine is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.Robert A. Smiley "Hexamethylenediamine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Synthesis Hexamethylenediamine was first reported by Theodor Curtius. It is produced by the hydrogenation of adiponitrile: :NC(CH2)4CN + 4 H2 → H2N(CH2)6NH2 The hydrogenation is conducted on molten adiponitrile diluted with ammonia, typical catalysts being based on cobalt and iron. The yield is good, but commercially significant side products are generated by virtue of reactivity of partially hydrogenated intermediates. These other products include 1,2-diaminocyclohexane, hexamethyleneimine, and the triamine bis(hexamethylenetriami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evonik Industries
Evonik Industries AG is a stock-listed German specialty chemicals company headquartered in Essen, North Rhine-Westphalia, Germany. It is the second largest chemicals company in Germany, and one of the largest specialty chemicals companies in the world. It is predominantly owned by the RAG Foundation and was founded on 12 September 2007 as a result of restructuring of the mining and technology group RAG. Evonik Industries united the business areas of chemicals, energy and real estate of RAG, while mining operations continue to be carried out by RAG. Since then, the energy and real estate business areas have been divested, with no share being held in the former and a minority share still being held in the latter. Its specialty chemicals business generates around 80% of sales in areas in which it holds leading market positions. Evonik Industries employs about 37,000 people and carries out activities in more than 100 countries. The operating activities are organized into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adipic Acid
Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Preparation and reactivity Adipic acid is produced from a mixture of cyclohexanone and cyclohexanol called KA oil, the abbreviation of ketone-alcohol oil. The KA oil is oxidized with nitric acid to give adipic acid, via a multistep pathway. Early in the reaction, the cyclohexanol is converted to the ketone, releasing nitrous acid: :HOC6H11 + HNO3 → OC(CH2)5 + HNO2 + H2O Among its many reactions, the cyclohexanone is nitrosated, setting the stage for the scission of the C-C bond: :HNO2 + HNO3 → NO+NO3− + H2O :OC6H10 + NO+ → OC6H9-2-NO + H+ Side produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are precursors to nylon. Cyclohexyl () is the alkyl substituent of cyclohexane and is abbreviated Cy. Production Modern On an industrial scale, cyclohexane is produced by hydrogenation of benzene in the presence of a Raney nickel catalyst. Producers of cyclohexane account for approximately 11.4% of global demand for benzene. The reaction is highly exothermic, with ΔH(500 K) = -216.37 kJ/mol. Dehydrogenation commenced noticeably above 300 °C, reflecting the favorable entropy for dehydrogenation. : Early Unlike benzene, cyclohexane is not found in natural resources such as coal. For this reason, early investigators synthesized their cyclohex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamines
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive. In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine. Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry. Aliphatic diamines Linear * 1 carbon: methylenediamine (diaminomethane) of theoretical interest only * 2 carbons: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA. File:Ethylene_diamine.png, Ethylenediamine * 3 carbons: 1,3-diaminopropane (propane-1,3-diamine) * 4 carbons: putrescine (butane-1,4-diamine) * 5 carbons: cadaverine (pentane-1,5-diamine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |