|
Trifluoromethyl Alcohol
Trifluoromethanol is a synthetic organic compound with the formula . It is also referred to as perfluoromethanol or trifluoromethyl alcohol. The compound is the simplest perfluoroalcohol. The substance is a colorless gas, which is unstable at room temperature. Synthesis Like all primary and secondary perfluoroalcohols, trifluoromethanol eliminates hydrogen fluoride in an endothermic reaction and forms carbonyl fluoride. : (I) At temperatures in the range of −120 °C, trifluoromethanol can be prepared from trifluoromethyl hypochlorite and hydrogen chloride: : (II) In this reaction, the recombination of a partially positively charged chlorine atom (in trifluoromethyl hypochlorite) with a partially negatively charged chlorine atom (in hydrogen chloride) is used as elemental chlorine. The undesired products, by-products chlorine, hydrogen chloride, and chlorotrifluoromethane, can be removed by evaporation at −110 °C. Trifluoromethanol has a melting point of −82&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluoroalcohol
Fluoroalcohols are organofluorine compounds consisting of an alcohol functional group with at least one C-F bond. These compounds often have distinctive solvent properties. Perfluoroalcohols Most primary and secondary perfluoroalcohols are unstable, for example trifluoromethanol eliminates hydrogen fluoride, forming carbonyl fluoride. This reaction is reversible. : Stable perfluorinated alcohols include nonafluoro-tert-butyl alcohol ((CF3)3COH) and pentafluorophenol (C6F5OH). Partially fluorinated alcohols Numerous partially fluorinated alcohols are known and have useable stabilities. Trifluoroethanol and hexafluoroisopropanol are used as solvents in research.{{cite journal , doi=10.1038/s41570-017-0088, title=Hexafluoroisopropanol as a Highly Versatile Solvent , year=2017 , last1=Colomer , first1=Ignacio , last2=Chamberlain , first2=Anna E. R. , last3=Haughey , first3=Maxwell B. , last4=Donohoe , first4=Timothy J. , journal=Nature Reviews Chemistry , volume=1 , issue=11 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
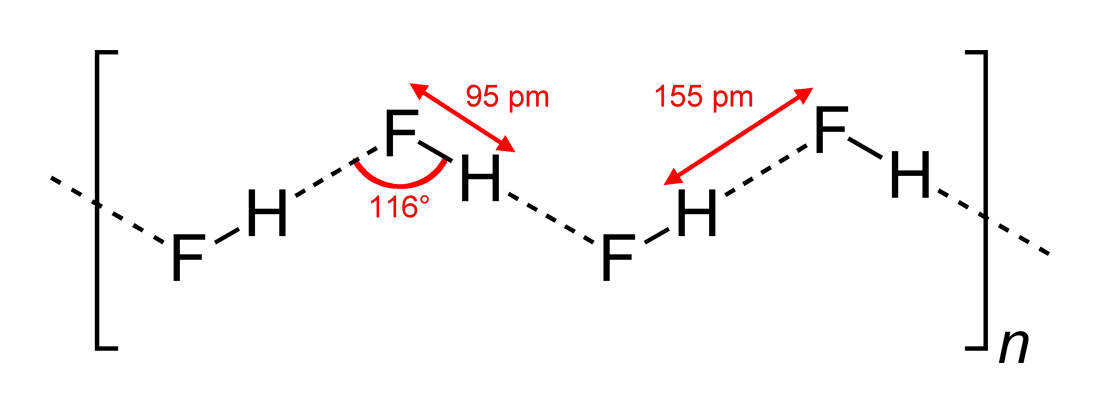
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothermic Reaction
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015). '' Principle of Modern Chemistry'', Brooks Cole. p. 617. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. The term was coined by 19th-century French chemist Marcellin Berthelot. The term ''endothermic'' comes from the Greek ἔνδον (''endon'') meaning 'within' and θερμ- (''therm'') meaning 'hot' or 'warm'. An endothermic process may be a chemical process, such as dissolving ammonium nitrate () in water (), or a physical process, such as the melting of ice cubes. The opposite of an endothermic process is an exoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Fluoride
Carbonyl fluoride is a chemical compound with the formula . It is a carbon oxohalide. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with ''C''2v symmetry, bond lengths of 1.174 Å (C=O) and 1.312 Å (C–F), and an F–C–F bond angle of 108.0°. Preparation and properties Carbonyl fluoride is usually produced as a decomposition product of fluorinated hydrocarbons in the thermal decomposition thereof, for example from trifluoromethanol or tetrafluoromethane in the presence of water: : Carbonyl fluoride can also be prepared by reaction of phosgene with hydrogen fluoride and the fluorination of carbon monoxide, although the latter tends to result in over-fluorination to carbon tetrafluoride. The fluorination of carbon monoxide with silver difluoride is convenient: : Carbonyl fluoride is unstable in the presence of water, hydrolyzing to carbon dioxide and hydrogen fluoride Hydrogen fluoride (fluorane) is an Inorganic chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethyl Hypochlorite
The trifluoromethyl group is a functional group that has the formula . The naming of is group is derived from the methyl group (which has the formula ), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane , 1,1,1-trifluoroethane , and hexafluoroacetone . Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from 1928, although resea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a Polar-covalent bond, polar covalent bond. The chlorine atom is much more Electronegativity, electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large Molecular dipole moment, dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoromethane
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive, nontoxic chlorofluorocarbon (CFC) and also a mixed halomethane. It is a man-made substance used primarily as a refrigerant. When released into the environment, CFC-13 has a high ozone depletion potential, and long atmospheric lifetime. Only a few other greenhouse gases surpass CFC-13 in global warming potential (GWP). The IPCC AR5 reported that CFC-13's atmospheric lifetime was 640 years. Production CFC-13like all chlorofluorocarbon compoundscontains atoms of carbon (C), chlorine (Cl), and fluorine (F). It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride: :CCl4 + 3 HF → CClF3 + 3 HCl This reaction can also produce trichlorofluoromethane (CCl3F), dichlorodifluoromethane (CCl2F2) and tetrafluoromethane (CF4). Montreal Protocol Following the unanimous ratification of the 1987 Montreal Protocolin response t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, Volatility (chemistry), volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a Precursor (chemistry), precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl ''tert''-butyl ether, methyl benzoate, anisole, peroxyacids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroantimonic Acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This mixture is a superacid stronger than pure sulfuric acid, by many orders of magnitude, according to its Hammett acidity function. It even protonates some hydrocarbons to afford pentacoordinate carbocations ( carbonium ions). Like its precursor hydrogen fluoride, it attacks glass, but can be stored in containers lined with PTFE (Teflon) or PFA. Chemical composition Fluoroantimonic acid is formed by combining hydrogen fluoride and antimony pentafluoride: :SbF5 + 2 HF + H2F+ The speciation (i.e., the inventory of components) of fluoroantimonic acid is complex. Spectroscopic measurements show that fluoroantimonic acid consists of a mixture of HF-solvated protons, – (such as ). Thus, the formula "" is a convenient but oversimplified approximation of the true composition. Nevertheless, the extreme acidity of this mixt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Fluoride
Sodium fluoride (NaF) is an inorganic compound with the formula . It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2022, it was the 221st most commonly prescribed medication in the United States, with more than 1million prescriptions. It is also used in metallurgy and in medical imaging. Uses Dental caries Fluoride salts are often added to municipal drinking water (as well as to certain food products in some countries) for the purpose of maintaining dental health. The fluoride enhances the strength of teeth by the formation of fluorapatite, a naturally occurring component of tooth enamel. Although sodium fluoride is used to fluoridate water and is the standard by which other water-fluoridation compounds are gauged, hexafluorosilicic acid (H2SiF6) and its salt sodium hexafluorosilicate (Na2Si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Environ
Environ or environs may refer to: * Environ (Loft), a New York performance space * Ramboll Environ, or ''ENVIRON'', a consulting firm in Arlington, Virginia * ''Environs'' (journal), a student-run law review covering environmental subjects * Environs, or surroundings Surroundings, or environs is an area around a given physical or geographical point or place. The exact definition depends on the field. Surroundings can also be used in geography (when it is more precisely known as vicinity, or vicinage) and ..., the area around a given physical or geographical point or place See also * * * Environment (other) {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |