|
Tricarboxylic Acids
A tricarboxylic acid is an organic chemistry, organic carboxylic acid that contain three carboxyl group, carboxyl functional groups (−COOH). A well-known example is citric acid. Promient examples Some prominent substituted tricarboxylic acids Citric acid, is used in the Citric Acid Cycle, citric acid cyclealso known as the ''tricarboxylic acid'' (''TCA'') ''cycle'' or ''Krebs cycle''which is fundamental to all Aerobic organism, aerobic organisms. Nitrilotriacetic acid (NTA) is a chelating agent for Ca2+, Co2+, Cu2+, and Fe3+. See also * Citric acid cycle (tricarboxylic acid cycle) * Dicarboxylic acid * Mellitic acid References Literature *{{cite journal , title = The Tricarboxylic Acid Cycle, an Ancient Metabolic Network with a Novel Twist. , author = Ryan J. Mailloux, Robin Bériault, Joseph Lemire, Ranji Singh, Daniel R. Chénier, Robert D. Hamel, Vasu D. Appanna , year = 2007 , journal = PLOS ONE , volume = 2 , issue = 8 , pages = e690 , doi = 10.1371/journa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agaric Acid Structural Formula V1
An agaric () is a type of fungal fruiting body characterized by the presence of a pileus (cap) that is clearly differentiated from the stipe (stalk), with lamellae (gills) on the underside of the pileus. It is a type of mushroom (or toadstool), the diverse group of agarics being lumped together as gilled mushrooms. "Agaric" can also refer more generally to any basidiomycete species characterized by an agaric-type fruiting body. Etymology Originally, agaric meant 'tree-fungus' (after Latin ''agaricum''); however, that changed with the Linnaean interpretation in 1753 when Linnaeus used the generic name ''Agaricus'' for gilled mushrooms. Taxonomy Most species of agarics belong to the order Agaricales in the subphylum Agaricomycotina. The exceptions, where agarics have evolved independently, feature largely in the orders Russulales, Boletales, Hymenochaetales, and several other groups of basidiomycetes. Old systems of classification placed all agarics in the Agaricales and som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
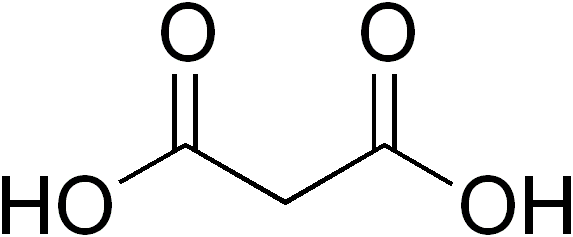
Dicarboxylic Acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are usually colorless solids. A wide variety of dicarboxylic acids are used in industry. Adipic acid, for example, is a precursor to certain kinds of nylon. A wide variety of dicarboxylic acids are found in nature. Aspartic acid and glutamic acid are two amino acids found in all life. Succinic and fumaric acids are essential for metabolism. A large inventory of derivatives are known including many mono- and diesters, amides, etc. Partial list of saturated dicarboxylic acids Some common or illustrative examples : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid Cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-CoA Redox, oxidation. The energy released is available in the form of Adenosine triphosphate, ATP. The Hans Krebs (biochemist), Krebs cycle is used by organisms that generate energy via Cellular respiration, respiration, either anaerobic respiration, anaerobically or aerobic respiration, aerobically (organisms that Fermentation, ferment use different pathways). In addition, the cycle provides precursor (chemistry), precursors of certain amino acids, as well as the reducing agent nicotinamide adenine dinucleotide, NADH, which are used in other reactions. Its central importance to many Metabolic pathway, biochemical pathways suggests that it was one of the earliest metabolism components. Even though it is branded as a "cycle", it is not necessa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agent
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity. The word ''chelation'' is derived from Greek χηλή, ''chēlē'', meaning "claw"; the ligands lie around the central atom like the claws of a crab. The term ''chelate'' () was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or ''chele'' (Greek) of the crab or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings." Chelation is useful in applications such as providing nutritional supplements, in ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrilotriacetic Acid
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid. Its conjugate base nitrilotriacetate is used as a chelating agent for Ca2+, Co2+, Cu2+, and Fe3+. Production and use Nitrilotriacetic acid is commercially available as the free acid and as the sodium salt. It is produced from ammonia, formaldehyde, and sodium cyanide or hydrogen cyanide. NTA is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct. Older routes to NTA included alkylation of ammonia with chloroacetic acid and oxidation of triethanolamine. Coordination chemistry and applications The conjugate base of NTA is a tripodal tetradentate trianionic ligand, forming coordination compounds with a variety of metal ions. Like EDTA, its sodium salt is used for water softening to remove Ca2+. For this purpose, NTA is a replacement for triphosphate, which once was widely used in detergents, and cleansers, but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerobic Organism
An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment. The ability to exhibit aerobic respiration may yield benefits to the aerobic organism, as aerobic respiration yields more energy than anaerobic respiration. Energy production of the cell involves the synthesis of ATP by an enzyme called ATP synthase. In aerobic respiration, ATP synthase is coupled with an electron transport chain in which oxygen acts as a terminal electron acceptor. In July 2020, marine biologists reported that aerobic microorganisms (mainly), in " quasi-suspended animation", were found in organically poor sediments, up to 101.5 million years old, 250 feet below the seafloor in the South Pacific Gyre (SPG) ("the deadest spot in the ocean"), and could be the longest-living life forms ever found. Types * Obligate aerobes need oxygen to grow. In a process known as cellular respiration, these organisms use oxygen to oxidize substrates (for example sugars and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid Cycle
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle, or TCA cycle (tricarboxylic acid cycle)—is a series of chemical reaction, biochemical reactions that release the energy stored in nutrients through acetyl-CoA Redox, oxidation. The energy released is available in the form of Adenosine triphosphate, ATP. The Hans Krebs (biochemist), Krebs cycle is used by organisms that generate energy via Cellular respiration, respiration, either anaerobic respiration, anaerobically or aerobic respiration, aerobically (organisms that Fermentation, ferment use different pathways). In addition, the cycle provides precursor (chemistry), precursors of certain amino acids, as well as the reducing agent nicotinamide adenine dinucleotide, NADH, which are used in other reactions. Its central importance to many Metabolic pathway, biochemical pathways suggests that it was one of the earliest metabolism components. Even though it is branded as a "cycle", it is not necessa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid
Citric acid is an organic compound with the formula . It is a Transparency and translucency, colorless Weak acid, weak organic acid. It occurs naturally in Citrus, citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid Commodity chemicals, are manufactured every year. It is used widely as acidifier, flavoring, preservative, and chelating agent. A citrate is a derivative of citric acid; that is, the salt (chemistry), salts, esters, and the polyatomic ion, polyatomic anion found in solutions and salts of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When citrate anion, trianion is part of a salt, the formula of the citrate trianion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably Citrus, citrus fruits. Lemons and Lime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimesic Acid
Trimesic acid, also known as benzene-1,3,5-tricarboxylic acid, is an organic compound with the formula C6H3(CO2H)3. It is one of three isomers of benzenetricarboxylic acid. A colorless solid, trimesic acid has some commercial value as a precursor to some plasticizer A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture. Plasticizer ...s. Trimesic acid can be combined with ''para''-hydroxypyridine to make a water-based gel, stable up to 95 °C. Trimesic acid crystallizes from water to form a hydrogen-bonded hydrated network with wide unidimensional empty channels. See also * Trimellitic acid (1,2,4-benzenetricarboxylic acid) * Hemimellitic acid (1,2,3-benzenetricarboxylic acid) References Benzoic acids Tricarboxylic acids Carboxylic acid-based porous polymer monomers {{OrganicAcid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |