|
Tricarbon Monoxide
Tricarbon monoxide C3O is a reactive radical (chemistry), radical oxocarbon molecule found in space, and which can be made as a transient substance in the laboratory. It can be trapped in an inert gas matrix or made as a short lived gas. C3O can be classified as a ketene or an oxocumulene a kind of heterocumulene. Natural occurrence C3O has been detected by its microwave spectrum in the dark cold Taurus Molecular Cloud One and also in the protostar Elias 18. The route to produce this is speculated to be: :HC + CO2 → HC3O+ + CO :HC3O+ → C3O + H+ or :C2 + CO → C3O which is more favourable at lower temperatures. The related C3S is more abundant in dark molecular clouds, even though oxygen is 20 times more common than sulfur. The difference is due to the higher rate of formation and that C3S is less polar. Production C3O can be produced by heating Meldrum's acid. This also produces acetone, carbon monoxide and carbon dioxide. R. L. DeKock and W. Waltner were the first to id ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Suboxide
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula and structure . Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons , which also includes carbon dioxide () and pentacarbon dioxide (). Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions. The substance was discovered in 1873 by Benjamin Brodie by subjecting carbon monoxide to an electric current. He claimed that the product was part of a series of "oxycarbons" with formulas , namely , , , , …, and to have identified the last two; however, only is known. In 1891 Marcellin Berthelot observed that heating pure carbon monoxide at about 550 °C created small amounts of carbon dioxide but no trace of carbon, and assumed that a carbon-rich oxide was created instead, which he named "sub-oxide". He assumed it was the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanuric Acid
Cyanuric acid or 1,3,5-triazine-2,4,6-triol is a chemical compound with the formula (CNOH)3. Like many industrially useful chemicals, this triazine has many synonyms. This white, odorless solid finds use as a precursor or a component of bleaches, disinfectants, and herbicides. In 1997, worldwide production was 160 000 tonnes.Klaus Huthmacher, Dieter Most "Cyanuric Acid and Cyanuric Chloride" Ullmann's Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. doi 10.1002/14356007.a08 191 Properties and synthesis Properties Cyanuric acid can be viewed as the cyclic trimer of the elusive species cyanic acid, HOCN. The ring can readily interconvert between several structures via lactam-lactim tautomerism. Although the triol tautomer may have aromatic character, the keto form predominates in solution. The hydroxyl (-OH) groups assume phenolic character. Deprotonation with base affords a series of cyanurate salts: : (O)NHsub>3 ⇌ (O)NHsub>2 (O)Nsup>− + H+ (pKa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uracil
Uracil () (symbol U or Ura) is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine (T). Uracil is a demethylated form of thymine. Uracil is a common and naturally occurring pyrimidine derivative. The name "uracil" was coined in 1885 by the German chemist Robert Behrend, who was attempting to synthesize derivatives of uric acid. Originally discovered in 1900 by Alberto Ascoli, it was isolated by hydrolysis of yeast nuclein; it was also found in bovine thymus and spleen, herring sperm, and wheat germ. It is a planar, unsaturated compound that has the ability to absorb light. Based on 12C/13C isotopic ratios of organic compounds found in the Murchison meteorite, it is believed that uracil, xanthine, and related molecules can also be formed extraterrestrially. Data from the Cassini mission, orbitin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor alkaline. The body uses it in many processes, most notably nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important raw material for the chemical industry. In 1828 Friedrich Wöhler discovered that urea can be produced from inorganic starting materials, which was an important conceptual milesto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin. In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds, with a nonbonded electron pair on the approximately tetrahedral sulfur atom. Synthesis and production Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Dimethyl sulfoxide is produced industrially from dimethyl sulfide, a by-product of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
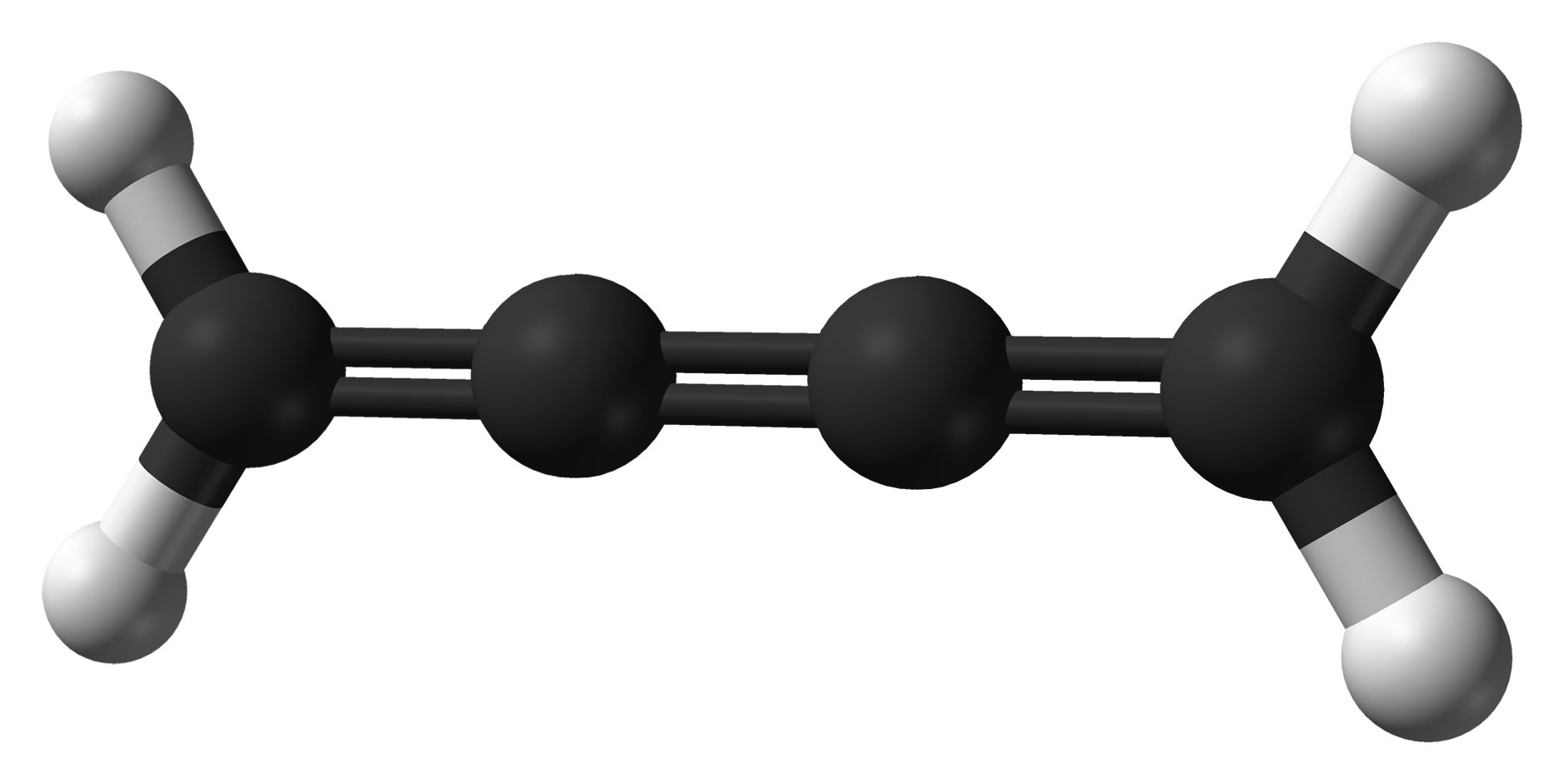
Cumulene
In organic chemistry, a cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular clouds and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide. Synthesis The first reported synthesis of a butatriene is that of tetraphenylbutatriene in 1921. The most common synthetic method for butatriene synthesis is based on reductive coupling of a geminal dihalo vinylidene. Tetraphenylbutatriene was reported sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylenes
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and in blue hydrocarbon flames. Diatomic carbon is the second simplest form of carbon after atomic carbon, and is an intermediate participator in the genesis of fullerenes. Properties C2 is a component of carbon vapor. One paper estimates that carbon vapor is around 28% diatomic, but theoretically this depends on the temperature and pressure. Electromagnetic properties The electrons in diatomic carbon are distributed among the molecular orbitals according to the Aufbau principle to produce unique quantum states, with corresponding energy levels. The state with the lowest energy level, or ground s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophosgene
Thiophosgene is a red liquid with the formula . It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses. Preparation is prepared in a two-step process from carbon disulfide. In the first step, carbon disulfide is chlorinated to give trichloromethanesulfenyl chloride (perchloromethyl mercaptan), : : The chlorination must be controlled as excess chlorine converts trichloromethanesulfenyl chloride into carbon tetrachloride Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVAC .... Steam distillation separates the trichloromethanesulfenyl chloride, a rare sulfenyl chloride, and hydrolyzes the sulfur monochloride. Reduction of trichloromethanesulfenyl chloride produces thiophosgene: : Tin and dihydroanthracene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyro'' "fire", "heat", "fever" and ''lysis'' "separating". Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.''Burning of wood'' , InnoFireWood's website. Accessed on 2010-02-06. In general, pyrolysis of organic substances produces volatile products and leaves char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly |