|
Transition Metal Sulfito Complex
Transition metal sulfito complexes are coordination compounds containing sulfite (SO32-) as a ligand. The inventory is large. Few sulfito complexes have commercial applications, but sulfite is a substrate for the molybdoenzyme sulfite oxidase. Bonding modes In principle, sulfite can bond to metal ions via S or O. To some extent, the sulfito ligand resembles nitrito (), which can bind through N or O. Monodentate, ''S''-bonded sulfites are more common than O-bonded sulfito ligands. ''S''-Bonded sulfite is a soft ligand with a strongly trans labilizing effect as indicated by the rapid aquation of to give . In some cases, sulfite serves as a bridging ligand forming M-SO2-O-M’ linkages. Examples * (tetren = ) * (M = Rh, Co, en = ethylenediamine Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Co(ethylenediamine)2(SO3)N3-3D-balls
CO or variants may refer to: Chemistry * Carbon monoxide (CO), a colorless, odorless, and tasteless gas * Carbonyl group, composed of a carbon atom double-bonded to an oxygen atom: C=O * Cobalt, a chemical element, symbol Co Computing and telecommunications * .co (second-level domain), the Internet second-level domain meaning "commercial" * .co, the Internet country code top-level domain (ccTLD) for Colombia * Commitment ordering (CO), a concurrency control technique for databases * Telephone exchange, or central office (CO) Mathematics * Cofunction, or Co, in trigonometry * Cuboctahedron, a uniform polyhedron People * Nguyễn Hữu Có (1925–2012), Vietnamese general * Conrado Co (born 1940), Filipino badminton player * Alfredo Co (born 1949), Filipino Sinologist * Atoy Co (born 1951), Filipino actor and basketball coach * Leonard Co (1953–2010), Filipino botanist * Nando Có (born 1973), Bissau-Guinean footballer * Kenedy Có (born 1998), Bissau-Guinean foot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Compound
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the periodic table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
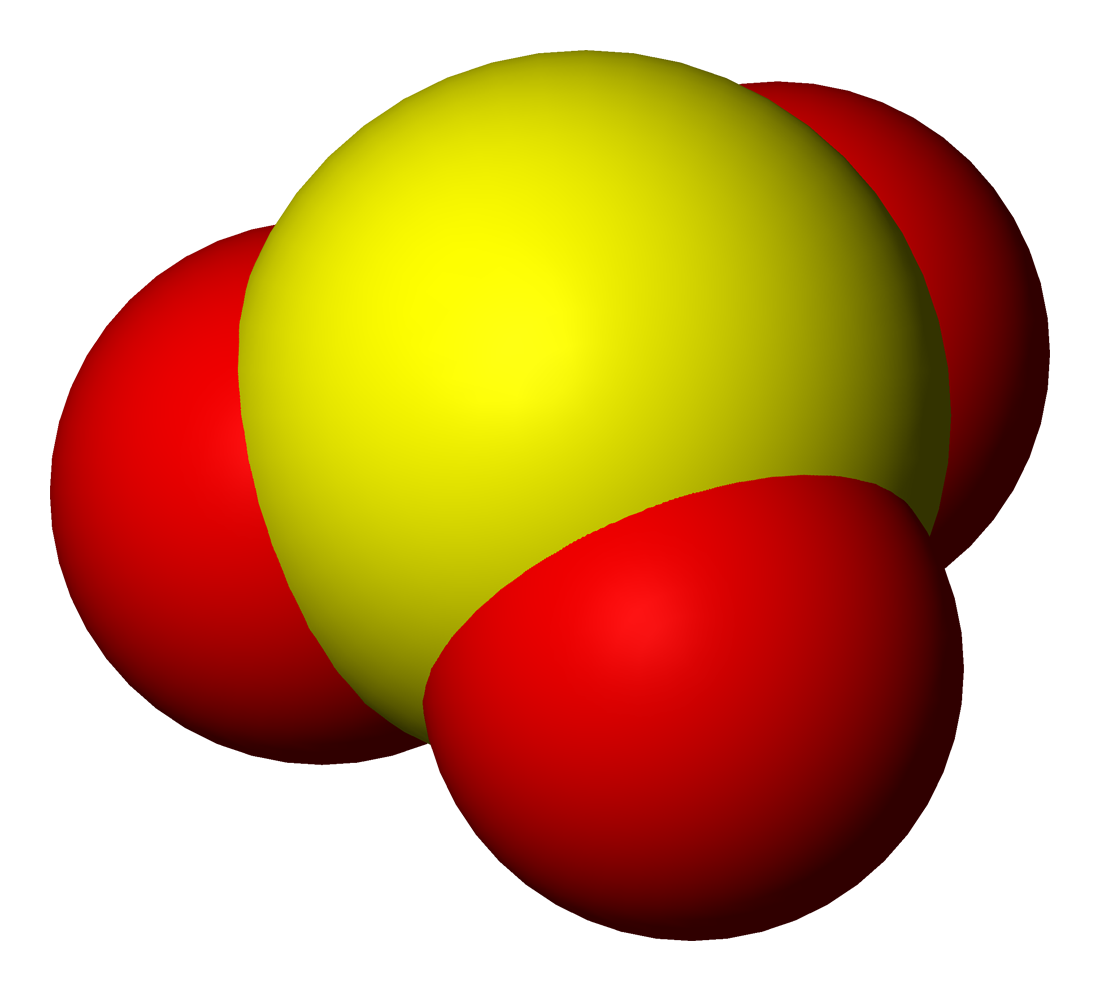
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structure predicted by VSEPR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite Oxidase
Sulfite oxidase () is an enzyme in the mitochondria of all eukaryotes, with exception of the yeasts. It oxidizes sulfite to sulfate and, via cytochrome c, transfers the electrons produced to the electron transport chain, allowing generation of ATP in oxidative phosphorylation. This is the last step in the metabolism of sulfur-containing compounds and the sulfate is excreted. Sulfite oxidase is a metallo-enzyme that utilizes a molybdopterin cofactor and a heme group (in the case of animals). It is one of the cytochromes ''b''5 and belongs to the enzyme super-family of molybdenum oxotransferases that also includes DMSO reductase, xanthine oxidase, and nitrite reductase. In mammals, the expression levels of sulfite oxidase is high in the liver, kidney, and heart, and very low in spleen, brain, skeletal muscle, and blood. Structure As a homodimer, sulfite oxidase contains two identical subunits with an N-terminal domain and a C-terminal domain. These two domains are connec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Nitrite Complex
In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite () ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle. Structure and bonding Bonding modes Three linkage isomers are common for nitrite ligands, O-bonded, N-bonded, and bidentate O,O-bonded. The former two isomers have been characterized for the pentamminecobalt(III) system, i.e. and , referred to as N-nitrito and O-nitrito, respectively. These two forms are sometimes called nitro and nitrito. These isomers can be interconverted in some complexes. An example of chelating nitrite is – "bipy" is the bidentate ligand bipyridine, 2,2′-bipyridyl. This bonding mode is sometimes described as κ2O,O-.. The kinetically-favored O-bonded isomer converts to . In its reaction with ferric porphyrin complexes, nitrite gives the O-bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HSAB Theory
HSAB is an acronym for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining the stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical species. 'Hard' applies to species which are small, have high charge states (the charge criterion applies mainly to acids, to a lesser extent to bases), and are weakly polarizable. 'Soft' applies to species which are big, have low charge states and are strongly polarizable. The theory is used in contexts where a qualitative, rather than quantitative, description would help in understanding the predominant factors which drive chemical properties and reactions. This is especially so in transition metal chemistry, where numerous experiments have been done to determine the relative ordering of ligands and transition metal ions in terms of their hardness and softness. HSAB theory is also useful in predicting the products of metathesis react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aquation
Aquation is the chemical reaction involving "incorporation of one or more integral molecules of water" with or without displacement of other atoms or groups. The term is typically employed to refer to reactions of metal complexes where an anion is displaced by water. For example, bromopentaamminecobalt(III) undergoes the following aquation reaction to give a metal aquo complex:{{Greenwood&Earnshaw2nd : o(NH3)5Brsup>2+ + H2O → o(NH3)5(H2O)sup>3+ + Br− This aquation reaction is catalyzed both by acid and by base. Acid catalysis involves protonation of the bromide, converting it to a better leaving group. Base hydrolysis proceeds by the SN1cB mechanism, which begins with deprotonation of an ammonia ligand. See also *Hydration reaction In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylenediamine
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines. Synthesis Ethylenediamine is produced industrially by treating 1,2-dichloroethane with ammonia under pressure at 180 °C in an aqueous medium (EDC process): : In this reaction hydrogen chloride is generated, which forms a salt with the amine. The amine is liberated by addition of sodium hydroxide and can then be recovered by fractional distillation. Diethylenetriamine (DETA) and triethylenetetramine (TETA) are formed as by-products. Another industrial route to ethylenediamine involves the reaction of ethanolamine and ammonia:Hans-Jürgen Arpe, Industrielle Organische Chemie, 6. Auflage (2007), Seite 275, Wiley VCH : Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(PtCl2(SO3)2H)3-
Platinum(II) chloride describes the inorganic compounds with the formula Pt Cl2. They are precursor used in the preparation of other platinum compounds. Platinum(II) chloride exists in two crystalline forms ( polymorphs), but the main properties are somewhat similar: dark brown, insoluble in water, diamagnetic, and odorless. Structure The structures of PtCl2 and PdCl2 are similar. These dichlorides exist in both polymeric, or "α", and hexameric, or "β" structures. The β form converts to the α form at 500 °C. In the β form, the Pt-Pt distances are 3.32–3.40 Å, indicative of some bonding between the pairs of metals. In both forms of PtCl2, each Pt center is four-coordinate, being surrounded by four chloride ligands. Complementarily, each Cl center is two-coordinate, being connected to two platinum atoms. The structure of α-PtCl2 is reported to be disordered and contain edge- and/or corner-sharing square-planar PtCl4 units. Preparation β-PtCl2 is prepared by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |